Search for drugs:
Typing the drug name to query
RALTEGRAVIR
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- At a dose 1.33 times the maximum approved recommended dose (and peak concentrations 1.25-fold higher than the maximum approved dose), raltegravir does not prolong the QT interval or PR interval to any clinically relevant extent.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
4
24088
Other ADRs
12355
38369232
Odds Ratio = 0.516
Drug Property Information
ATC Code(s):
- J05AJ01 - raltegravir
- J05AJ -
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
- J05AR16 - raltegravir
- J05AR - "Antivirals for treatment of HIV infections, combinations"
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:RALTEGRAVIR POTASSIUM
Active Ingredient UNII:43Y000U234
Drugbank ID:DB06817
PubChem Compound:54671008
CTD ID: D000068898
CAS Number:518048-05-0
Dosage Form(s):granule, for suspension; tablet, chewable; tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 800.0 mg/day J05AJ01
Chemical Structure: 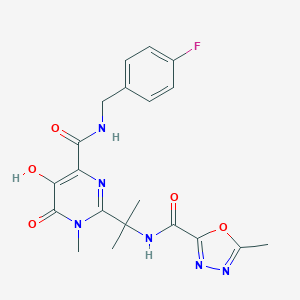

SMILE Code:
CN1C(=O)C(O)=C(N=C1C(C)(C)NC(=O)C1=NN=C(C)O1)C(=O)NCC1=CC=C(F)C=C1
CN1C(=O)C(O)=C(N=C1C(C)(C)NC(=O)C1=NN=C(C)O1)C(=O)NCC1=CC=C(F)C=C1
Reference
1: Interferon-free therapy with direct acting antivirals for HCV/HIV-1 co-infected Japanese patients with inherited bleeding disorders.
[Uemura Haruka,Tsukada Kunihisa,Mizushima Daisuke,Aoki Takahiro,Watanabe Koji,Kinai Ei,Teruya Katsuji,Gatanaga Hiroyuki,Kikuchi Yoshimi,Sugiyama Masaya,Mizokami Masashi,Oka Shinichi]PLoS One,2017 Oct 18;12(10):e0186255. PMID: 29045448
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.