Search for drugs:
Typing the drug name to query
PALBOCICLIB
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- The effect of palbociclib on the QT interval corrected for heart rate (QTc) was evaluated using time-matched electrocardiograms (ECGs) evaluating the change from baseline and corresponding pharmacokinetic data in 77 patients with breast cancer. Palbociclib had no large effect on QTc (i.e., >20 ms) at 125 mg once daily for 21 consecutive days followed by 7 days off treatment to comprise a complete cycle of 28 days.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
33
24059
Other ADRs
130409
38251178
Odds Ratio = 0.403
Drug Property Information
ATC Code(s):
- L01EF01 - palbociclib
- L01EF -
- L01E -
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:PALBOCICLIB
Active Ingredient UNII:G9ZF61LE7G
Drugbank ID:DB09073
PubChem Compound:5330286
CTD ID: C500026
PharmGKB:PA166153469
CAS Number:571190-30-2
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 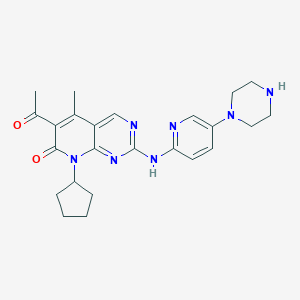

SMILE Code:
CC(=O)C1=C(C)C2=CN=C(NC3=NC=C(C=C3)N3CCNCC3)N=C2N(C2CCCC2)C1=O
CC(=O)C1=C(C)C2=CN=C(NC3=NC=C(C=C3)N3CCNCC3)N=C2N(C2CCCC2)C1=O
Reference
1: Real-world safety of palbociclib in breast cancer patients in the United States: a new user cohort study.
[Beachler Daniel C,de Luise Cynthia,Jamal-Allial Aziza,Yin Ruihua,Taylor Devon H,Suzuki Ayako,Lewis James H,Freston James W,Lanes Stephan]BMC Cancer,2021 Jan 25;21(1):97. PMID: 33494720
2: CDK4/6 inhibitors in breast cancer: differences in toxicity profiles and impact on agent choice. A systematic review and meta-analysis.
[Onesti Concetta E,Jerusalem Guy]Expert Rev Anticancer Ther,2021 Mar;21(3):283-298. PMID: 33233970
3: Clinical Pharmacokinetics and Pharmacodynamics of the Cyclin-Dependent Kinase 4 and 6 Inhibitors Palbociclib, Ribociclib, and Abemaciclib.
[Groenland Stefanie L,Martínez-Chávez Alejandra,van Dongen Marloes G J,Beijnen Jos H,Schinkel Alfred H,Huitema Alwin D R,Steeghs Neeltje]Clin Pharmacokinet,2020 Dec;59(12):1501-1520. PMID: 33029704
4: Comparative efficacy of palbociclib, ribociclib and abemaciclib for ER+ metastatic breast cancer: an adjusted indirect analysis of randomized controlled trials.
[Petrelli Fausto,Ghidini Antonio,Pedersini Rebecca,Cabiddu Mary,Borgonovo Karen,Parati Maria Chiara,Ghilardi Mara,Amoroso Vito,Berruti Alfredo,Barni Sandro]Breast Cancer Res Treat,2019 Apr;174(3):597-604. PMID: 30659432
5: Management of adverse events during cyclin-dependent kinase 4/6 (CDK4/6) inhibitor-based treatment in breast cancer.
[Thill Marc,Schmidt Marcus]Ther Adv Med Oncol,2018 Sep 3;10:1758835918793326. PMID: 30202447
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.