Search for drugs:
Typing the drug name to query
GUANFACINE
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- In a thorough QT study, the administration of two dose levels of immediate-release guanfacine (4 mg and 8 mg) produced concentrations approximately 2 to 4 times the concentrations observed with the maximum recommended dose of guanfacine of 0.12 mg/kg. Guanfacine was not shown to prolong the QTc interval to any clinically relevant extent.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
77
24015
Other ADRs
5915
38375672
Odds Ratio = 20.803
Drug Property Information
ATC Code(s):
- C02AC02 - guanfacine
- C02AC - Imidazoline receptor agonists
- C02A - "ANTIADRENERGIC AGENTS, CENTRALLY ACTING"
- C02 - ANTIHYPERTENSIVES
- C - CARDIOVASCULAR SYSTEM
Active Ingredient:GUANFACINE HYDROCHLORIDE
Active Ingredient UNII:PML56A160O
Drugbank ID:DB01018
PubChem Compound:3519
CTD ID:D016316
PharmGKB:PA449825
CAS Number:29110-47-2
Dosage Form(s):tablet, extended release
Route(s) Of Administrator:oral
Daily Dose:
- 3.0 mg/day C02AC02
Chemical Structure: 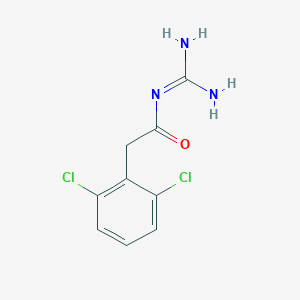

SMILE Code:
NC(=N)NC(=O)CC1=C(Cl)C=CC=C1Cl
NC(=N)NC(=O)CC1=C(Cl)C=CC=C1Cl
Reference
1: Use of ECG restitution (beat-to-beat QT-TQ interval analysis) to assess arrhythmogenic risk of QTc prolongation with guanfacine.
[Fossa Anthony A,Zhou Meijian,Robinson Antoine,Purkayastha Jaideep,Martin Patrick]Ann Noninvasive Electrocardiol,2014 Nov;19(6):582-94. PMID: 25200912
2: Alpha-2 agonists for attention-deficit/hyperactivity disorder in youth: a systematic review and meta-analysis of monotherapy and add-on trials to stimulant therapy.
[Hirota Tomoya,Schwartz Shimon,Correll Christoph U]J Am Acad Child Adolesc Psychiatry,2014 Feb;53(2):153-73. PMID: 24472251
3: Risk of serious cardiovascular problems with medications for attention-deficit hyperactivity disorder.
[Martinez-Raga Jose,Knecht Carlos,Szerman Nestor,Martinez María I]CNS Drugs,2013 Jan;27(1):15-30. PMID: 23160939
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.