Search for drugs:
Typing the drug name to query
SELPERCATINIB
DIR Classification
Classification:Moderate-DIQT concern
Severity Score:3.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
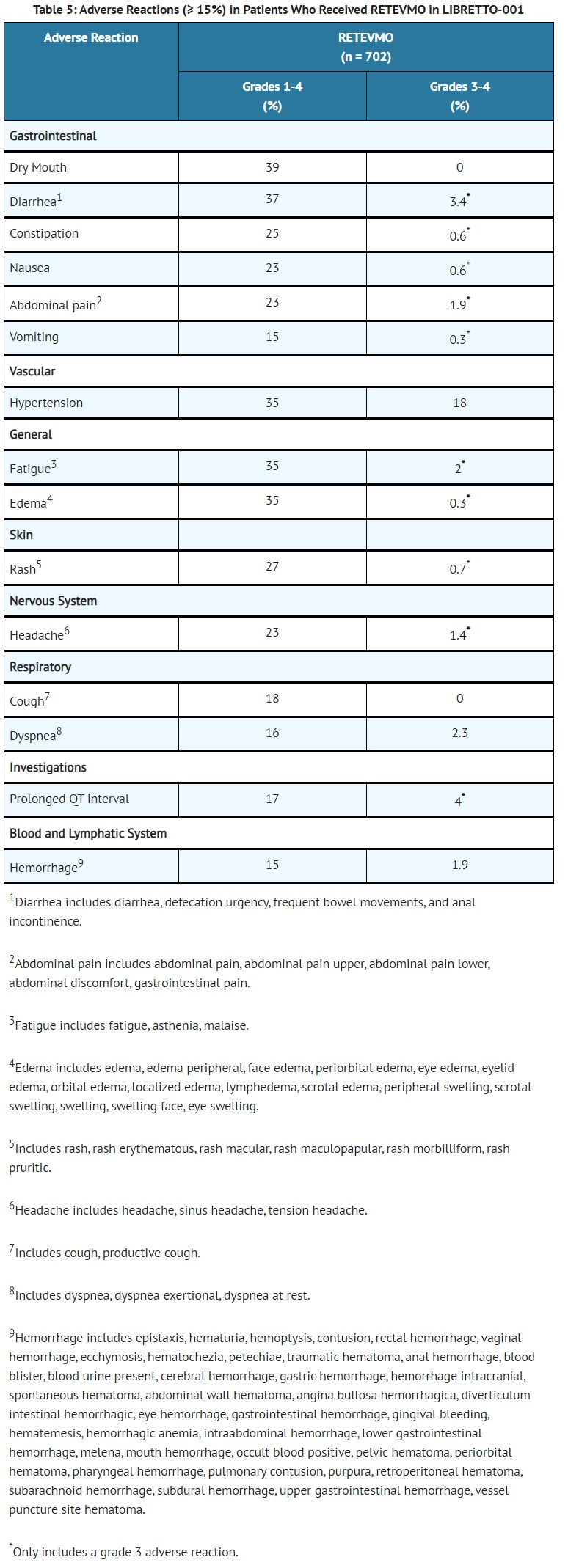
- RETEVMO can cause concentration-dependent QT interval prolongation [see Clinical Pharmacology (12.2)]. An increase in QTcF interval to >500 ms was measured in 6% of patients and an increase in the QTcF interval of at least 60 ms over baseline was measured in 15% of patients [see Adverse Reactions (6.1)]. RETEVMO has not been studied in patients with clinically significant active cardiovascular disease or recent myocardial infarction.
- Monitor patients who are at significant risk of developing QTc prolongation, including patients with known long QT syndromes, clinically significant bradyarrhythmias, and severe or uncontrolled heart failure. Assess QT interval, electrolytes and TSH at baseline and periodically during treatment, adjusting frequency based upon risk factors including diarrhea. Correct hypokalemia, hypomagnesemia and hypocalcemia prior to initiating RETEVMO and during treatment.
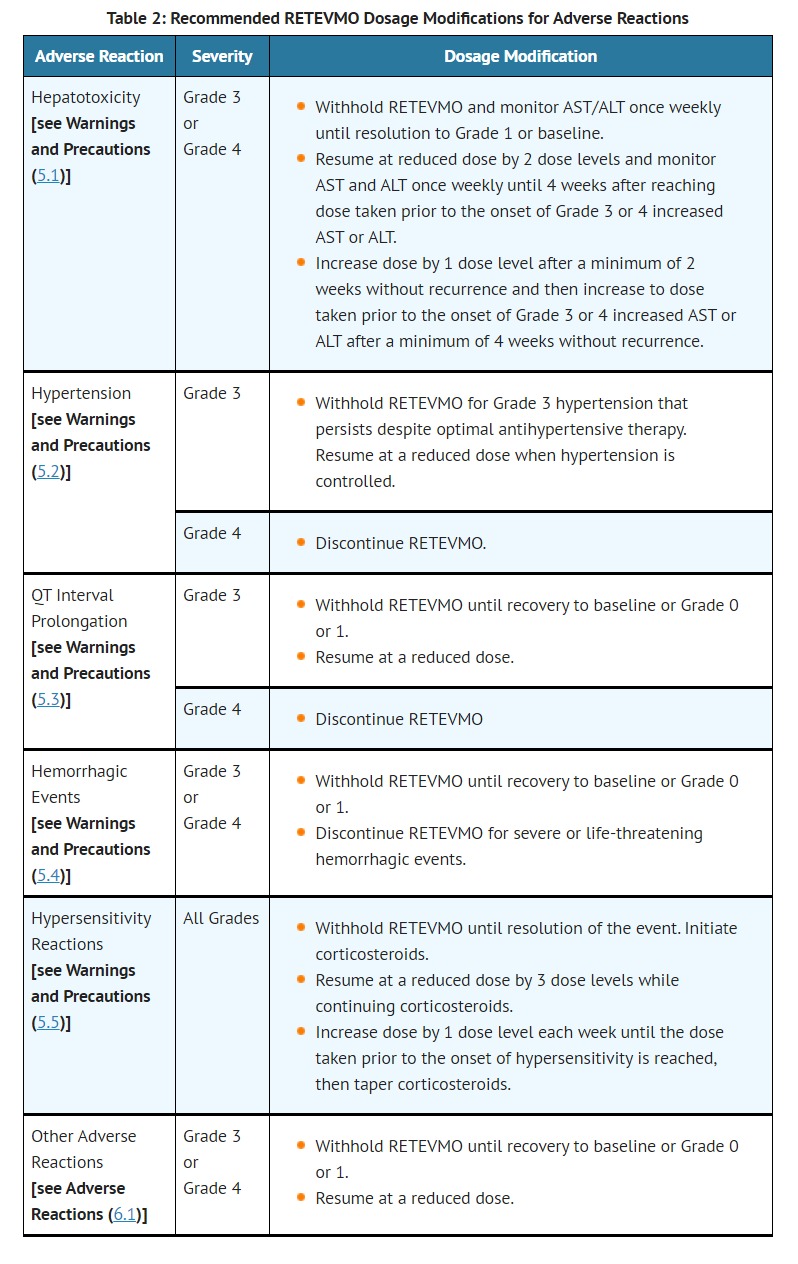
- Monitor the QT interval more frequently when RETEVMO is concomitantly administered with strong and moderate CYP3A inhibitors or drugs known to prolong QTc interval. Withhold and dose reduce or permanently discontinue RETEVMO based on the severity [see Dosage and Administration (2.5)].
- DRUG INTERACTIONS
- Effects of Other Drugs on RETEVMO
- Strong and Moderate CYP3A Inhibitors
- Concomitant use of RETEVMO with a strong or moderate CYP3A inhibitor increases selpercatinib plasma concentrations [see Clinical Pharmacology (12.3)], which may increase the risk of RETEVMO adverse reactions, including QTc interval prolongation.
- Avoid concomitant use of strong and moderate CYP3A inhibitors with RETEVMO. If concomitant use of strong and moderate CYP3A inhibitors cannot be avoided, reduce the RETEVMO dosage and monitor the QT interval with ECGs more frequently [see Dosage and Administration (2.6), Warning and Precautions (5.3)].
- DOSAGE AND ADMINISTRATION
- ADVERSE REACTIONS
- Clinical Trials Experience
- Dosage interruptions due to an adverse reaction occurred in 42% of patients who received RETEVMO. Adverse reactions requiring dosage interruption in ≥ 2% of patients included ALT increased, AST increased, hypertension, diarrhea, pyrexia, and QT prolongation.
- Dose reductions due to an adverse reaction occurred in 31% of patients who received RETEVMO. Adverse reactions requiring dosage reductions in ≥ 2% of patients included ALT increased, AST increased, QT prolongation and fatigue.
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- The effect of RETEVMO on the QTc interval was evaluated in a thorough QT study in healthy subjects. The largest mean increase in QTc is predicted to be 10.6 msec (upper 90% confidence interval: 12.1 msec) at the mean steady-state maximum concentration (Cmax) observed in patients after administration of 160 mg twice daily. The increase in QTc was concentration-dependent.
- PATIENT COUNSELING INFORMATION
- QT Prolongation
- Advise patients that RETEVMO can cause QTc interval prolongation and to inform their healthcare provider if they have any QTc interval prolongation symptoms, such as syncope [see Warnings and Precautions (5.3)].
- What are the possible side effects of RETEVMO?
- RETEVMO may cause serious side effects, including:
- Heart rhythm changes (QT prolongation) can occur and may be serious. RETEVMO may cause very slow, very fast or irregular heartbeats. Tell your healthcare provider right away if you get any of the following symptoms:
- loss of consciousness
- fainting
- dizziness
- a change in the way your heart beats (heart palpitations)
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
0
38381587
Odds Ratio = N/A
Drug Property Information
ATC Code(s):
Active Ingredient:selpercatinib
Active Ingredient UNII:CEGM9YBNGD
Drugbank ID:DB15685
PubChem Compound:N/ADIR Classification
CTD ID:C000656166
CAS Number:2152628-33-4
Dosage Form(s):capsule
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 

SMILE Code:
COC1=NC=C(CN2C3CC2CN(C3)C2=CC=C(C=N2)C2=CC(OCC(C)(C)O)=CN3N=CC(C#N)=C23)C=C1
COC1=NC=C(CN2C3CC2CN(C3)C2=CC=C(C=N2)C2=CC(OCC(C)(C)O)=CN3N=CC(C#N)=C23)C=C1
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.