Search for drugs:
Typing the drug name to query
DEUTETRABENAZINE
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QTc Prolongation
- AUSTEDO may prolong the QT interval, but the degree of QT prolongation is not clinically significant when AUSTEDO is administered within the recommended dosage range [see Clinical Pharmacology (12.2)].
- AUSTEDO should be avoided in patients with congenital long QT syndrome and in patients with a history of cardiac arrhythmias. Certain circumstances may increase the risk of the occurrence of torsade de pointes and/or sudden death in association with the use of drugs that prolong the QTc interval, including (1) bradycardia; (2) hypokalemia or hypomagnesemia; (3) concomitant use of other drugs that prolong the QTc interval; and (4) presence of congenital prolongation of the QT interval.
- ADVERSE REACTIONS
- The following serious adverse reactions are discussed in greater detail in other sections of the labeling:
- Depression and Suicidality in Patients with Huntington’s disease [see Warnings and Precautions (5.1)]
- QTc Prolongation [see Warnings and Precautions (5.3)]
- Neuroleptic Malignant Syndrome (NMS) [see Warnings and Precautions (5.4)]
- Akathisia, Agitation, and Restlessness [see Warnings and Precautions (5.5)]
- Parkinsonism [see Warnings and Precautions (5.6)]
- Sedation and Somnolence [see Warnings and Precautions (5.7)]
- Hyperprolactinemia [see Warnings and Precautions (5.8)]
- Binding to Melanin-Containing Tissues [see Warnings and Precautions (5.9)]
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- At the maximum recommended dose, AUSTEDO does not prolong the QT interval to any clinically relevant extent. An exposure-response analysis on QTc prolongation from a study in extensive or intermediate (EM) and poor CYP2D6 metabolizers (PM) showed that a clinically-relevant effect can be excluded at exposures following single doses of 24 and 48 mg of AUSTEDO.
- PATIENT COUNSELING INFORMATION
- Prolongation of the QTc Interval
- Inform patients to consult their physician immediately if they feel faint, lose consciousness, or have heart palpitations [see Warnings and Precautions (5.3)]. Advise patients to inform physicians that they are taking AUSTEDO before any new drug is taken.
- MEDICATION GUIDE
- Before taking AUSTEDO, tell your healthcare provider about all of your medical conditions, including if you:
- have emotional or mental problems (for example, depression, nervousness, anxiety, anger, agitation, psychosis, previous suicidal thoughts or suicide attempts).
- have liver disease.
- have an irregular heart rhythm or heartbeat (QT prolongation, cardiac arrhythmia) or a heart problem called congenital long QT syndrome.
- have low levels of potassium or magnesium in your blood (hypokalemia or hypomagnesemia).
- have breast cancer or a history of breast cancer.
- are pregnant or plan to become pregnant. It is not known if AUSTEDO can harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if AUSTEDO passes into breast milk.
- AUSTEDO can cause serious side effects, including:
- Irregular heartbeat (QT prolongation). AUSTEDO increases your chance of having certain changes in the electrical activity in your heart. These changes can lead to a dangerous abnormal heartbeat. Taking AUSTEDO with certain medicines may increase this chance.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
5
24087
Other ADRs
4292
38377295
Odds Ratio = 1.857
Drug Property Information
ATC Code(s):
Active Ingredient:DEUTETRABENAZINE
Active Ingredient UNII:P341G6W9NB
Drugbank ID:DB12161
PubChem Compound:73442840
CTD ID: C000609690
CAS Number:1392826-25-3
Dosage Form(s):kit; tablet, coated
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 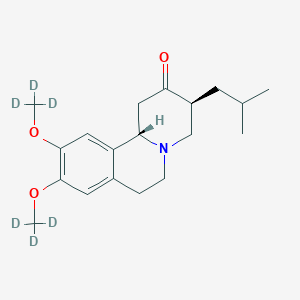

SMILE Code:
[2H]C([2H])([2H])OC1=CC2=C(C=C1OC([2H])([2H])[2H])[C@@H]1CC(=O)[C@@H](CC(C)C)CN1CC2
[2H]C([2H])([2H])OC1=CC2=C(C=C1OC([2H])([2H])[2H])[C@@H]1CC(=O)[C@@H](CC(C)C)CN1CC2
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.