Search for drugs:
Typing the drug name to query
KETOCONAZOLE
DIR Classification
Classification:Most-DIQT concern
Severity Score:5.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- BOXED WARNING
- QT Prolongation and Drug Interactions Leading to QT Prolongation
- Co-administration of the following drugs with ketoconazole is contraindicated: dofetilide, quinidine, pimozide, cisapride, methadone, disopyramide, dronedarone, ranolazine. Ketoconazole can cause elevated plasma concentrations of these drugs and may prolong QT intervals, sometimes resulting in life-threatening ventricular dysrhythmias such as torsades de pointes. See CONTRAINDICATIONS, WARNINGS, and PRECAUTIONS: DRUG INTERACTIONS sections.
- WARNINGS
- QT Prolongation and Drug Interactions Leading to QT Prolongation
- Ketoconazole can prolong the QT interval. Co-administration of the following drugs with ketoconazole is contraindicated: dofetilide, quinidine, pimozide, cisapride, methadone, disopyramide, dronedarone, ranolazine. Ketoconazole can cause elevated plasma concentrations of these drugs which may prolong the QT interval, sometimes resulting in life-threatening ventricular dysrhythmias such as torsades de pointes.
- PRECAUTIONS
- Drug Interactions
- Drugs that may have their plasma concentrations increased by ketoconazole
- Ketoconazole can inhibit the metabolism of drugs metabolized by CYP3A4 and can inhibit the drug transport by P-glycoprotein, which may result in increased plasma concentrations of these drugs and/or their active metabolite(s) when they are administered with ketoconazole. These elevated plasma concentrations may increase or prolong both therapeutic and adverse effects of these drugs. CYP3A4-metabolized drugs known to prolong the QT interval may be contraindicated with ketoconazole tablets, since the combination may lead to ventricular tachyarrhythmias, including occurrences of torsade de pointes, a potentially fatal arrhythmia.
- Drugs that may have their plasma concentrations increased by ketoconazole
- Ketoconazole can inhibit the metabolism of drugs metabolized by CYP3A4 and can inhibit the drug transport by P-glycoprotein, which may result in increased plasma concentrations of these drugs and/or their active metabolite(s) when they are administered with ketoconazole. These elevated plasma concentrations may increase or prolong both therapeutic and adverse effects of these drugs. CYP3A4-metabolized drugs known to prolong the QT interval may be contraindicated with ketoconazole tablets, since the combination may lead to ventricular tachyarrhythmias, including occurrences of torsade de pointes, a potentially fatal arrhythmia.

- CONTRAINDICATIONS
- Drug Interactions
- Coadministration of a number of CYP3A4 substrates such as dofetilide, quinidine cisapride and pimozide is contraindicated with ketoconazole tablets. Coadministration with ketoconazole can cause elevated plasma concentrations of these drugs and may increase or prolong both therapeutic and adverse effects to such an extent that a potentially serious adverse reaction may occur. For example, increased plasma concentrations of some of these drugs can lead to QT prolongation and sometimes resulting in life-threatening ventricular tachyarrhythmias including occurrences of torsades de pointes, a potentially fatal arrhythmia. (See PRECAUTIONS: DRUG INTERACTIONS.)
- Additionally, the following other drugs are contraindicated with ketoconazole tablets: methadone, disopyramide, dronedarone, ergot alkaloids such as dihydroergotamine, ergometrine, ergotamine, methylergometrine, irinotecan, lurasidone, oral midazolam, alprazolam, triazolam, felodipine, nisoldipine, ranolazine, tolvaptan, eplerenone, lovastatin, simvastatin and colchicine. (See PRECAUTIONS: DRUG INTERACTIONS.)
- CLINICAL PHARMACOLOGY
- Electrocardiogram
- Pre-clinical electrophysiological studies have shown that ketoconazole inhibits the rapidly activating component of the cardiac delayed rectifier potassium current, prolongs the action potential duration, and may prolong the QT c interval. Data from some clinical PK/PD studies and drug interaction studies suggest that oral dosing with ketoconazole at 200 mg twice daily for 3 to 7 days can result in an increase of the QT c interval: a mean maximum increase of about 6 to 12 msec was seen at ketoconazole peak plasma concentrations about 1 to 4 hours after ketoconazole administration.
- MEDICATION GUIDE
- changes in the electrical activity of your heart called QT prolongation. QT prolongation can cause irregular heart beats that can be life threatening. This can happen when ketoconazole tablets are taken with certain medicines, such as dofetilide, quinidine, pimozide, cisapride, methadone, disopyramide, dronedarone, and ranolazine. Talk to your healthcare provider about other medicines you are taking before you start taking ketoconazole tablets. Tell your healthcare provider right away if you feel faint, lightheaded, dizzy, or feel your heart beating irregularly or fast. These may be symptoms related to QT prolongation.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
16
24076
Other ADRs
8461
38373126
Odds Ratio = 3.014
Drug Property Information
ATC Code(s):
- J02AB02 - ketoconazole
- J02AB - Imidazole derivatives
- J02A - ANTIMYCOTICS FOR SYSTEMIC USE
- J02 - ANTIMYCOTICS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
- H02CA03 - ketoconazole
- H02CA - Anticorticosteroids
- H02C - ANTIADRENAL PREPARATIONS
- H02 - CORTICOSTEROIDS FOR SYSTEMIC USE
- H - "SYSTEMIC HORMONAL PREPARATIONS, EXCL. "
- G01AF11 - ketoconazole
- G01AF - Imidazole derivatives
- G01A - "ANTIINFECTIVES AND ANTISEPTICS, EXCL. COMBINATIONS "
- G01 - GYNECOLOGICAL ANTIINFECTIVES AND ANTISEPTICS
- G - GENITO URINARY SYSTEM AND SEX HORMONES
- D01AC08 - ketoconazole
- D01AC - Imidazole and triazole derivatives
- D01A - ANTIFUNGALS FOR TOPICAL USE
- D01 - ANTIFUNGALS FOR DERMATOLOGICAL USE
- D - DERMATOLOGICALS
Active Ingredient:KETOCONAZOLE
Active Ingredient UNII:R9400W927I
Drugbank ID:DB01026
PubChem Compound:3823
CTD ID:D007654
PharmGKB:PA450146
CAS Number:65277-42-1
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 200.0 mg/day J02AB02
- 400.0 mg/day G01AF11
Chemical Structure: 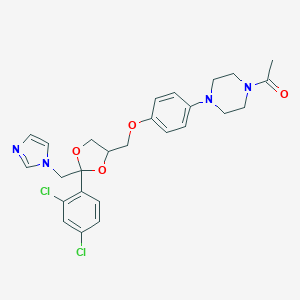

SMILE Code:
CC(=O)N1CCN(CC1)C1=CC=C(OCC2COC(CN3C=CN=C3)(O2)C2=CC=C(Cl)C=C2Cl)C=C1
CC(=O)N1CCN(CC1)C1=CC=C(OCC2COC(CN3C=CN=C3)(O2)C2=CC=C(Cl)C=C2Cl)C=C1
Reference
1: Utilization of the chronic atrioventricular block cynomolgus monkey as an in vivo model to evaluate drug interaction-associated torsade de pointes.
[Goto Ai,Sakamoto Kengo,Hagiwara-Nagasawa Mihoko,Kambayashi Ryuichi,Chiba Koki,Nunoi Yoshio,Izumi-Nakaseko Hiroko,Matsumoto Akio,Sugiyama Atsushi]J Pharmacol Sci,2020 Apr;142(4):172-175. PMID: 31982331
2: Efficacy and safety of levoketoconazole in the treatment of endogenous Cushing's syndrome (SONICS): a phase 3, multicentre, open-label, single-arm trial.
[Fleseriu Maria,Pivonello Rosario,Elenkova Atanaska,Salvatori Roberto,Auchus Richard J,Feelders Richard A,Geer Eliza B,Greenman Yona,Witek Przemyslaw,Cohen Fredric,Biller Beverly M K]Lancet Diabetes Endocrinol,2019 Nov;7(11):855-865. PMID: 31542384
3: Progress in the Consideration of Possible Sex Differences in Drug Interaction Studies.
[Naidoo Panjasaram,Chetty Manoranjenni]Curr Drug Metab,2019;20(2):114-123. PMID: 30488793
4: Persistence of a Posaconazole-Mediated Drug-Drug Interaction With Ranolazine After Cessation of Posaconazole Administration: Impact of Obesity and Implications for Patient Safety.
[Chow Christina R,Harmatz Jerold S,Ryan Michael J,Greenblatt David J]J Clin Pharmacol,2018 Nov;58(11):1436-1442. PMID: 29749631
5: Torsade de pointes and systemic azole antifungal agents: Analysis of global spontaneous safety reports.
[Salem M,Reichlin T,Fasel D,Leuppi-Taegtmeyer A]Glob Cardiol Sci Pract,2017 Jun 30;2017(2):11. PMID: 29644223
6: Drug-induced Inhibition and Trafficking Disruption of ion Channels: Pathogenesis of QT Abnormalities and Drug-induced Fatal Arrhythmias.
[Cubeddu Luigi X]Curr Cardiol Rev,2016;12(2):141-54. PMID: 26926294
7: No Untoward Effect of Long-Term Ketoconazole Administration on Electrocardiographic QT Interval in Patients with Cushing's Disease.
[De Martin Martina,Toja Paola Maria,Goulene Karine,Radaelli Piero,Cavagnini Francesco,Stramba-Badiale Marco,Pecori Giraldi Francesca]Basic Clin Pharmacol Toxicol,2016 Apr;118(4):279-83. PMID: 26386326
8: Effect of axitinib on the QT interval in healthy volunteers.
[Ruiz-Garcia Ana,Houk Brett E,Pithavala Yazdi K,Toh Melvin,Sarapa Nenad,Tortorici Michael A]Cancer Chemother Pharmacol,2015 Mar;75(3):619-28. PMID: 25589220
9: Predicting QTc Prolongation in Man From Only In Vitro Data.
[Leishman D J]CPT Pharmacometrics Syst Pharmacol,2014 Aug 20;3(8):e131. PMID: 25141222
10: Interaction Between Domperidone and Ketoconazole: Toward Prediction of Consequent QTc Prolongation Using Purely In Vitro Information.
[Mishra H,Polak S,Jamei M,Rostami-Hodjegan A]CPT Pharmacometrics Syst Pharmacol,2014 Aug 13;3(8):e130. PMID: 25116274
11: Potential interactions between HIV drugs, ritonavir and efavirenz and anticancer drug, nilotinib--a study in primary cultures of human hepatocytes that is applicable to HIV patients with cancer.
[Pillai Venkateswaran C,Parise Robert A,Christner Susan M,Rudek Michelle A,Beumer Jan H,Venkataramanan Raman]J Clin Pharmacol,2014 Nov;54(11):1272-9. PMID: 24846165
12: Lomitapide at supratherapeutic plasma levels does not prolong the Qtc interval--results from a TQT study with moxifloxacin and ketoconazole.
[Darpo Borje,Ferber Georg,Zhou Meijian,Sumeray Mark,Sager Philip]Ann Noninvasive Electrocardiol,2013 Nov;18(6):577-89. PMID: 24118671
13: Cutaneous leishmaniasis in Switzerland: first experience with species-specific treatment.
[Mosimann V,Neumayr A,Hatz C,Blum J A]Infection,2013 Dec;41(6):1177-82. PMID: 23835701
14: A randomized, crossover, placebo- and moxifloxacin-controlled study to evaluate the effects of bosutinib (SKI-606), a dual Src/Abl tyrosine kinase inhibitor, on cardiac repolarization in healthy adult subjects.
[Abbas Richat,Hug Bruce A,Leister Cathie,Sonnichsen Daryl]Int J Cancer,2012 Aug 1;131(3):E304-11. PMID: 22065400
15: Pharmacokinetic interaction between domperidone and ketoconazole leads to QT prolongation in healthy volunteers: a randomized, placebo-controlled, double-blind, crossover study.
[Boyce Malcolm J,Baisley Kathy J,Warrington Steven J]Br J Clin Pharmacol,2012 Mar;73(3):411-21. PMID: 21883386
16: Evaluation of terfenadine and ketoconazole-induced QT prolongation in conscious telemetered guinea pigs.
[Rajput Satyendra K,Singh Jitendra N,Sharma Shyam S]Pharmacol Rep,Jul-Aug 2010;62(4):683-8. PMID: 20885008
17: Iatrogenic QT Abnormalities and Fatal Arrhythmias: Mechanisms and Clinical Significance.
[Cubeddu Luigi X]Curr Cardiol Rev,2009 Aug;5(3):166-76. PMID: 20676275
18: Phase 1 pharmacokinetic and drug-interaction study of dasatinib in patients with advanced solid tumors.
[Johnson Faye M,Agrawal Shruti,Burris Howard,Rosen Lee,Dhillon Navneet,Hong David,Blackwood-Chirchir Anne,Luo Feng R,Sy Oumar,Kaul Sanjeev,Chiappori Alberto A]Cancer,2010 Mar 15;116(6):1582-91. PMID: 20108303
19: Inhibition of hERG channel trafficking: an under-explored mechanism for drug-induced QT prolongation.
[Yeung Kap-Sun,Meanwell Nicholas A]ChemMedChem,2008 Oct;3(10):1501-2. PMID: 18696522
20: Ritonavir 100 mg does not cause QTc prolongation in healthy subjects: a possible role as CYP3A inhibitor in thorough QTc studies.
[Sarapa N,Nickens D J,Raber S R,Reynolds R R,Amantea M A]Clin Pharmacol Ther,2008 Jan;83(1):153-9. PMID: 17581594
21: Detecting signals of drug-drug interactions in a spontaneous reports database.
[Thakrar Bharat T,Grundschober Sabine Borel,Doessegger Lucette]Br J Clin Pharmacol,2007 Oct;64(4):489-95. PMID: 17506784
22: Interaction between ketoconazole and domperidone and the risk of QT prolongation--important safety information.
[Medicines Control Council]S Afr Med J,2006 Jul;96(7):596. PMID: 16909179
23: Life-threatening bradyarrhythmia after massive azithromycin overdose.
[Tilelli John A,Smith Kathleen M,Pettignano Robert]Pharmacotherapy,2006 Jan;26(1):147-50. PMID: 16506357
24: Benefit-risk assessment of tolterodine in the treatment of overactive bladder in adults.
[Garely Alan D,Burrows Lara]Drug Saf,2004;27(13):1043-57. PMID: 15471509
25: Inhibition of cytochrome P450 3A: relevant drug interactions in gastroenterology.
[Sagir A,Schmitt M,Dilger K,Häussinger D]Digestion,2003;68(1):41-8. PMID: 12949438
26: The importance of the QT interval: a review of the literature.
[Elming H,Sonne J,Lublin H K F]Acta Psychiatr Scand,2003 Feb;107(2):96-101. PMID: 12534434
27: [Why is QT interval interesting?].
[Elming Hanne,Sonne Jesper,Lublin Henrik K F]Ugeskr Laeger,2002 Feb 4;164(6):750-4. PMID: 11851179
28: Drug interactions with cisapride: clinical implications.
[Michalets E L,Williams C R]Clin Pharmacokinet,2000 Jul;39(1):49-75. PMID: 10926350
29: Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition.
[Dresser G K,Spence J D,Bailey D G]Clin Pharmacokinet,2000 Jan;38(1):41-57. PMID: 10668858
30: Effects of H1 antihistamines on animal models of QTc prolongation.
[Gras J,Llenas J]Drug Saf,1999;21 Suppl 1:39-44; discussion 81-7. PMID: 10597867
31: Cardiotoxicity of histamine and the possible role of histamine in the arrhythmogenesis produced by certain antihistamines.
[Llenas J,Cardelús I,Heredia A,de Mora F,Gristwood R W]Drug Saf,1999;21 Suppl 1:33-8; discussion 81-7. PMID: 10597866
32: The QT interval and torsade de pointes.
[Moss A J]Drug Saf,1999;21 Suppl 1:5-10; discussion 81-7. PMID: 10597863
33: Blockade of HERG and Kv1.5 by ketoconazole.
[Dumaine R,Roy M L,Brown A M]J Pharmacol Exp Ther,1998 Aug;286(2):727-35. PMID: 9694927
34: Systemic antifungal agents. Drug interactions of clinical significance.
[Albengres E,Le Louët H,Tillement J P]Drug Saf,1998 Feb;18(2):83-97. PMID: 9512916
35: Combined use of astemizole and ketoconazole resulting in torsade de pointes.
[Tsai W C,Tsai L M,Chen J H]J Formos Med Assoc,1997 Feb;96(2):144-6. PMID: 9071844
36: Cardiotoxic and drug interaction profile of the second generation antihistamines ebastine and terfenadine in an experimental animal model of torsade de pointes.
[Hey J A,del Prado M,Kreutner W,Egan R W]Arzneimittelforschung,1996 Feb;46(2):159-63. PMID: 8720305
37: Torsades de pointes and long QT syndromes.
[Janeira L F]Am Fam Physician,1995 Oct;52(5):1447-53. PMID: 7572567
38: Torsades de pointes induced by nonantiarrhythmic drugs.
[Tran H T]Conn Med1994 May;58(5):291-5. PMID: 7915666
39: Torsade de pointes complicating treatment with astemizol.
[Kulkarni S M,Agarwal H K,Shaikh A A]Indian Heart J,May-Jun 1994;46(3):179-80. PMID: 7821942
40: Is QT interval prolongation harmful? A regulatory perspective.
[Botstein P]Am J Cardiol1993 Aug 26;72(6):50B-52B. PMID: 8256756
41: Variability of the QTc interval: impact on defining drug effect and low-frequency cardiac event.
[Morganroth J,Brown A M,Critz S,Crumb W J,Kunze D L,Lacerda A E,Lopez H]Am J Cardiol,1993 Aug 26;72(6):26B-31B. PMID: 8256752
42: Terfenadine-ketoconazole interaction. Pharmacokinetic and electrocardiographic consequences.
[Honig P K,Wortham D C,Zamani K,Conner D P,Mullin J C,Cantilena L R]JAMA,1993 Mar 24-31;269(12):1513-8. PMID: 8445813
43: Effect of concomitant administration of cimetidine and ranitidine on the pharmacokinetics and electrocardiographic effects of terfenadine.
[Honig P K,Wortham D C,Zamani K,Conner D P,Mullin J C,Cantilena L R]Eur J Clin Pharmacol,1993;45(1):41-6. PMID: 8405028
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.