Search for drugs:
Typing the drug name to query
TAZEMETOSTAT
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- The effect of orally administered TAZVERIK, at doses ranging from 100 mg to 1600 mg twice daily (0.125 to 2 times the approved recommended dosage) for 15 days, on the heart-rate corrected QT (QTc) interval was evaluated in a dose-finding study in 38 patients with advanced malignancies. Tazemetostat and its metabolite EPZ-6930 did not cause a large mean increase (i.e. >20 ms) on the QTc interval at the 800 mg twice daily dose. The largest mean increase (upper bound of 90% confidence interval) in QTc were 6.1 ms (8.5 ms) and 9.3 ms (12.5 ms) at a dose of 800 mg twice daily and 1600 mg twice daily, respectively.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
0
38381587
Odds Ratio = N/A
Drug Property Information
ATC Code(s):
Active Ingredient:tazemetostat hydrobromide
Active Ingredient UNII:6P89T5M073
Drugbank ID:DB12887
PubChem Compound:66558664
CTD ID:C000593333
CAS Number:1403254-99-8
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 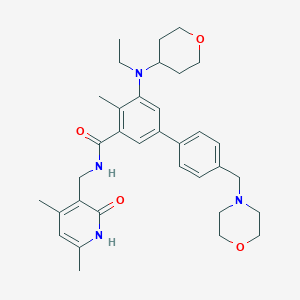

SMILE Code:
CCN(C1CCOCC1)C1=C(C)C(=CC(=C1)C1=CC=C(CN2CCOCC2)C=C1)C(=O)NCC1=C(C)C=C(C)NC1=O
CCN(C1CCOCC1)C1=C(C)C(=CC(=C1)C1=CC=C(CN2CCOCC2)C=C1)C(=O)NCC1=C(C)C=C(C)NC1=O
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.