Search for drugs:
Typing the drug name to query
ILOPERIDONE
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QT Prolongation
- In an open-label QTc study in patients with schizophrenia or schizoaffective disorder (n=160), iloperidone was associated with QTc prolongation of 9 msec at an iloperidone dose of 12 mg twice daily. The effect of iloperidone on the QT interval was augmented by the presence of CYP450 2D6 or 3A4 metabolic inhibition (paroxetine 20 mg once daily and ketoconazole 200 mg twice daily, respectively). Under conditions of metabolic inhibition for both 2D6 and 3A4, iloperidone tablets 12 mg twice daily was associated with a mean QTcF increase from baseline of about 19 msec.
- No cases of torsade de pointes or other severe cardiac arrhythmias were observed during the pre-marketing clinical program.
- The use of iloperidone tablets should be avoided in combination with other drugs that are known to prolong QTc including Class 1A (e.g., quinidine, procainamide) or Class III (e.g., amiodarone, sotalol) antiarrhythmic medications, antipsychotic medications (e.g., chlorpromazine, thioridazine), antibiotics (e.g., gatifloxacin, moxifloxacin), or any other class of medications known to prolong the QTc interval (e.g., pentamidine, levomethadyl acetate, methadone). Iloperidone tablets should also be avoided in patients with a known genetic susceptibility to congenital long QT syndrome and in patients with a history of cardiac arrhythmias.
- Certain circumstances may increase the risk of torsade de pointes and/or sudden death in association with the use of drugs that prolong the QTc interval, including (1) bradycardia; (2) hypokalemia or hypomagnesemia; (3) concomitant use of other drugs that prolong the QTc interval; and (4) presence of congenital prolongation of the QT interval; (5) recent acute myocardial infarction; and/or (6) uncompensated heart failure.
- Caution is warranted when prescribing iloperidone tablets with drugs that inhibit iloperidone metabolism [see DRUG INTERACTIONS (7.1)], and in patients with reduced activity of CYP2D6 [see CLINICAL PHARMACOLOGY (12.3)].
- It is recommended that patients being considered for iloperidone tablet treatment who are at risk for significant electrolyte disturbances have baseline serum potassium and magnesium measurements with periodic monitoring. Hypokalemia (and/or hypomagnesemia) may increase the risk of QT prolongation and arrhythmia. Iloperidone tablets should be avoided in patients with histories of significant cardiovascular illness, e.g., QT prolongation, recent acute myocardial infarction, uncompensated heart failure, or cardiac arrhythmia. Iloperidone tablets should be discontinued in patients who are found to have persistent QTc measurements >500 msec.
- If patients taking iloperidone tablets experience symptoms that could indicate the occurrence of cardiac arrhythmias, e.g., dizziness, palpitations, or syncope, the prescriber should initiate further evaluation, including cardiac monitoring.
- DRUG INTERACTIONS
- Drugs that Prolong the QT Interval
- Iloperidone tablets should not be used with any other drugs that prolong the QT interval [see WARNINGS AND PRECAUTIONS (5.3)].
- OVERDOSAGE
- Human Experience
- In pre-marketing trials involving over 3210 patients, accidental or intentional overdose of iloperidone tablets was documented in 8 patients ranging from 48 mg to 576 mg taken at once and 292 mg taken over a 3-day period. No fatalities were reported from these cases. The largest confirmed single ingestion of iloperidone was 576 mg; no adverse physical effects were noted for this patient. The next largest confirmed ingestion of iloperidone was 438 mg over a 4-day period; extrapyramidal symptoms and a QTc interval of 507 msec were reported for this patient with no cardiac sequelae. This patient resumed iloperidone tablet treatment for an additional 11 months. In general, reported signs and symptoms were those resulting from an exaggeration of the known pharmacological effects (e.g., drowsiness and sedation, tachycardia and hypotension) of iloperidone.
- [Management of Overdose]
- There is no specific antidote for iloperidone tablets. Therefore appropriate supportive measures should be instituted. In case of acute overdose, the physician should establish and maintain an airway and ensure adequate oxygenation and ventilation. Gastric lavage (after intubation, if patient is unconscious) and administration of activated charcoal together with a laxative should be considered. The possibility of obtundation, seizures or dystonic reaction of the head and neck following overdose may create a risk of aspiration with induced emesis. Cardiovascular monitoring should commence immediately and should include continuous ECG monitoring to detect possible arrhythmias. If antiarrhythmic therapy is administered, disopyramide, procainamide and quinidine should not be used, as they have the potential for QT-prolonging effects that might be additive to those of iloperidone. Similarly, it is reasonable to expect that the alpha-blocking properties of bretylium might be additive to those of iloperidone, resulting in problematic hypotension. Hypotension and circulatory collapse should be treated with appropriate measures such as intravenous fluids or sympathomimetic agents (epinephrine and dopamine should not be used, since beta stimulation may worsen hypotension in the setting of iloperidone-induced alpha blockade). In cases of severe extrapyramidal symptoms, anticholinergic medication should be administered. Close medical supervision should continue until the patient recovers.
- PATIENT COUNSELING INFORMATION
- QT Interval Prolongation
- Patients should be advised to consult their physician immediately if they feel faint, lose consciousness or have heart palpitations. Patients should be counseled not to take iloperidone tablets with other drugs that cause QT interval prolongation [see WARNINGS AND PRECAUTIONS (5.3)]. Patients should be told to inform physicians that they are taking iloperidone tablets before any new drug is taken.
- INDICATIONS AND USAGE
- Iloperidone tablets are indicated for the treatment of schizophrenia in adults.
- When deciding among the alternative treatments available for this condition, the prescriber should consider the finding that iloperidone tablets are associated with prolongation of the QTc interval [see WARNINGS AND PRECAUTIONS (5.3)]. Prolongation of the QTc interval is associated in some other drugs with the ability to cause torsade de pointes-type arrhythmia, a potentially fatal polymorphic ventricular tachycardia which can result in sudden death. In many cases this would lead to the conclusion that other drugs should be tried first. Whether iloperidone tablets will cause torsade de pointes or increase the rate of sudden death is not yet known. Patients must be titrated to an effective dose of iloperidone tablets. Thus, control of symptoms may be delayed during the first 1 to 2 weeks of treatment compared to some other antipsychotic drugs that do not require a similar titration. Prescribers should be mindful of this delay when selecting an antipsychotic drug for the treatment of schizophrenia [see DOSAGE AND ADMINISTRATION (2.1) and CLINICAL STUDIES (14)].
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
17
24075
Other ADRs
2074
38379513
Odds Ratio = 13.067
Drug Property Information
ATC Code(s):
- N05AX14 - iloperidone
- N05AX - Other antipsychotics
- N05A - ANTIPSYCHOTICS
- N05 - PSYCHOLEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:Iloperidone
Active Ingredient UNII:VPO7KJ050N
Drugbank ID:DB04946
PubChem Compound:71360
CTD ID:C081732
PharmGKB:PA161199368
CAS Number:133454-47-4
Dosage Form(s):kit; tablet
Route(s) Of Administrator:oral
Daily Dose:
- 18.0 mg/day N05AX14
Chemical Structure: 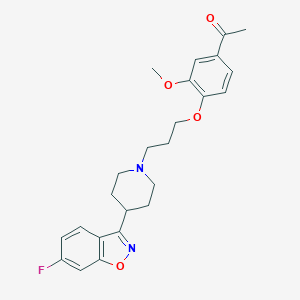

SMILE Code:
COC1=C(OCCCN2CCC(CC2)C2=NOC3=C2C=CC(F)=C3)C=CC(=C1)C(C)=O
COC1=C(OCCCN2CCC(CC2)C2=NOC3=C2C=CC(F)=C3)C=CC(=C1)C(C)=O
Reference
1: QT Prolongation, Torsades de Pointes, and Psychotropic Medications: A 5-Year Update.
[Beach Scott R,Celano Christopher M,Sugrue Alan M,Adams Caitlin,Ackerman Michael J,Noseworthy Peter A,Huffman Jeff C]Psychosomatics,Mar-Apr 2018;59(2):105-122. PMID: 29275963
2: Management of psychosis and agitation in medical-surgical patients who have or are at risk for prolonged QT interval.
[Ries Rose,Sayadipour Amirali]J Psychiatr Pract,2014 Sep;20(5):338-44. PMID: 25226194
3: QTc interval prolongation and torsade de pointes associated with second-generation antipsychotics and antidepressants: a comprehensive review.
[Hasnain Mehrul,Vieweg W Victor R]CNS Drugs,2014 Oct;28(10):887-920. PMID: 25168784
4: Safety profile of iloperidone in the treatment of schizophrenia.
[Dargani Navin V,Malhotra Anil K]Expert Opin Drug Saf,2014 Feb;13(2):241-6. PMID: 24206391
5: Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis.
[Leucht Stefan,Cipriani Andrea,Spineli Loukia,Mavridis Dimitris,Orey Deniz,Richter Franziska,Samara Myrto,Barbui Corrado,Engel Rolf R,Geddes John R,Kissling Werner,Stapf Marko Paul,Lässig Bettina,Salanti Georgia,Davis John M]Lancet,2013 Sep 14;382(9896):951-62. PMID: 23810019
6: Iloperidone for the treatment of schizophrenia.
[Bishop J R,Bishop D L]Drugs Today (Barc),2010 Aug;46(8):567-79. PMID: 20830317
7: Whole genome association study identifies polymorphisms associated with QT prolongation during iloperidone treatment of schizophrenia.
[Volpi S,Heaton C,Mack K,Hamilton J B,Lannan R,Wolfgang C D,Licamele L,Polymeropoulos M H,Lavedan C]Mol Psychiatry,2009 Nov;14(11):1024-31. PMID: 18521091
8: Four-week, double-blind, placebo- and ziprasidone-controlled trial of iloperidone in patients with acute exacerbations of schizophrenia.
[Cutler Andrew J,Kalali Amir H,Weiden Peter J,Hamilton Jennifer,Wolfgang Curt D]J Clin Psychopharmacol,2008 Apr;28(2 Suppl 1):S20-8. PMID: 18334909
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.