Search for drugs:
Typing the drug name to query
HYDROXYCHLOROQUINE SULFATE
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- PRECAUTIONS
- Drugs that prolong QT interval and other arrhythmogenic drugs: hydroxychloroquine sulfate tablets prolong the QT interval and should not be administered with other drugs that have the potential to induce cardiac arrhythmias. Also, there may be an increased risk of inducing ventricular arrhythmias if hydroxychloroquine sulfate tablets are used concomitantly with other arrhythmogenic drugs.
- OVERDOSAGE
- The 4-aminoquinoline compounds are very rapidly and completely absorbed after ingestion, and in accidental overdosage, or rarely with lower doses in hypersensitive patients, toxic symptoms may occur within 30 minutes. The symptoms of overdosage may include headache, drowsiness, visual disturbances, cardiovascular collapse, convulsions, hypokalemia, rhythm and conduction disorders including QT prolongation, torsades de pointes, ventricular tachycardia and ventricular fibrillation, followed by sudden potentially fatal respiratory and cardiac arrest. Treatment is symptomatic and must be prompt. Immediate gastric lavage until the stomach is completely emptied is indicated. After lavage, activated charcoal is introduced by the stomach tube within 30 minutes of ingestion of the drug may inhibit further intestinal absorption. To be effective, the dose of activated charcoal should be at least five times the estimated dose of hydroxychloroquine ingested.
- ADVERSE REACTIONS
- Cardiac disorders: Cardiomyopathy which may result in cardiac failure and in some cases a fatal outcome (see WARNINGS and OVERDOSAGE). Hydroxychloroquine sulfate tablets prolong the QT interval. Ventricular arrhythmias and torsade de pointes have been reported in patients taking hydroxychloroquine sulfate tablets (see OVERDOSAGE and DRUG INTERACTIONS).
- INDICATIONS AND USAGE
- Cardiac Effects, including Cardiomyopathy and QT prolongation: Postmarketing cases of life-threatening and fatal cardiomyopathy have been reported with use of hydroxychloroquine sulfate tablets as well as with use of chloroquine. Patients may present with atrioventricular block, pulmonary hypertension, sick sinus syndrome or with cardiac complications. ECG findings may include atrioventricular, right or left bundle branch block. Signs or symptoms of cardiac compromise have appeared during acute and chronic treatment. Clinical monitoring for signs and symptoms of cardiomyopathy is advised, including use of appropriate diagnostic tools such as ECG to monitor patients for cardiomyopathy during Hydroxychloroquine Sulfate Tablets therapy. Chronic toxicity should be considered when conduction disorders (bundle branch block/atrio-ventricular heart block) or biventricular hypertrophy are diagnosed. If cardiotoxicity is suspected, prompt discontinuation of hydroxychloroquine sulfate tablets may prevent lifethreatening complications.
- Hydroxychloroquine sulfate tablets prolong the QT interval. Ventricular arrhythmias and torsades de pointes have been reported in patients taking Hydroxychloroquine Sulfate Tablets (see OVERDOSAGE). Therefore, hydroxychloroquine sulfate tablets should not be administered with other drugs that have the potential to prolong the QT interval (see DRUG INTERACTIONS).
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
314
23778
Other ADRs
40091
38341496
Odds Ratio = 12.63
Drug Property Information
ATC Code(s):
- P01BA02 - hydroxychloroquine sulfate
- P01BA - Aminoquinolines
- P01B - ANTIMALARIALS
- P01 - ANTIPROTOZOALS
- P - "ANTIPARASITIC PRODUCTS, INSECTICIDES AND REPELLENTS"
Active Ingredient:HYDROXYCHLOROQUINE SULFATE
Active Ingredient UNII:8Q2869CNVH
Drugbank ID:DB01611
PubChem Compound:3652
CTD ID:D006886
PharmGKB:PA164777036
CAS Number:118-42-3
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 516.0 mg/day P01BA02
Chemical Structure: 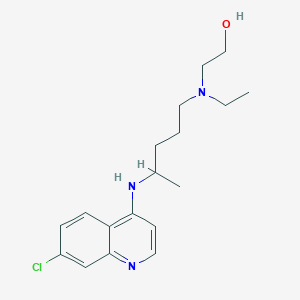

SMILE Code:
CCN(CCO)CCCC(C)NC1=C2C=CC(Cl)=CC2=NC=C1
CCN(CCO)CCCC(C)NC1=C2C=CC(Cl)=CC2=NC=C1
Reference
1: Hydroxychloroquine for the treatment and prophylaxis of COVID-19: The journey so far and the road ahead.
[Singh Harmanjit,Chauhan Prerna,Kakkar Ashish Kumar]Eur J Pharmacol,2021 Jan 5;890:173717. PMID: 33152333
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.