Search for drugs:
Typing the drug name to query
LINEZOLID
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- In a randomized, positive- and placebo-controlled crossover thorough QT study, 40 healthy subjects were administered a single ZYVOX 600 mg dose via a 1 hour IV infusion, a single ZYVOX 1,200 mg dose via a 1 hour IV infusion, placebo, and a single oral dose of positive control. At both the 600 mg and 1,200 mg ZYVOX doses, no significant effect on QTc interval was detected at peak plasma concentration or at any other time.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
74
24018
Other ADRs
25177
38356410
Odds Ratio = 4.694
Drug Property Information
ATC Code(s):
- J01XX08 - linezolid
- J01XX0 -
- J01XX - Other antibacterials
- J01X - OTHER ANTIBACTERIALS
- J01 - ANTIBACTERIALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:LINEZOLID
Active Ingredient UNII:ISQ9I6J12J
Drugbank ID:DB00601
PubChem Compound:441401
CTD ID:D000069349
PharmGKB:PA450233
CAS Number:165800-03-3
Dosage Form(s):injection, solution; suspension; tablet, film coated
Route(s) Of Administrator:intravenous; oral
Daily Dose:
- 1200.0 mg/day J01XX08
Chemical Structure: 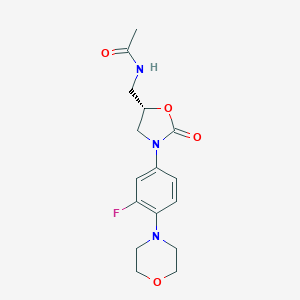

SMILE Code:
CC(=O)NC[C@H]1CN(C(=O)O1)C1=CC(F)=C(C=C1)N1CCOCC1
CC(=O)NC[C@H]1CN(C(=O)O1)C1=CC(F)=C(C=C1)N1CCOCC1
Reference
1: {'#text': 'Comparative Efficacy of the Novel Diarylquinoline TBAJ-587 and Bedaquiline against a Resistant Mutant in a Mouse Model of Tuberculosis.', 'i': 'Rv0678'}
[Xu Jian,Converse Paul J,Upton Anna M,Mdluli Khisimuzi,Fotouhi Nader,Nuermberger Eric L]Antimicrob Agents Chemother,2021 Mar 18;65(4):e02418-20. PMID: 33526488
2: Treatment of multidrug-resistant pulmonary tuberculosis with delamanid based on Japanese guideline recommendations.
[Okumura Masao,Yoshiyama Takashi,Ogata Hideo,Kurashima Atsuyuki,Yoshimori Kozo,Ohta Ken,Kudoh Shoji]Respir Investig,2020 Mar;58(2):110-116. PMID: 31838040
3: Long-Term Effects on QT Prolongation of Pretomanid Alone and in Combinations in Patients with Tuberculosis.
[Li Hanbin,Salinger David H,Everitt Daniel,Li Mengchun,Del Parigi Angelo,Mendel Carl,Nedelman Jerry R]Antimicrob Agents Chemother,2019 Sep 23;63(10):e00445-19. PMID: 31358590
4: Torsades de pointes and QT prolongation Associations with Antibiotics: A Pharmacovigilance Study of the FDA Adverse Event Reporting System.
[Teng Chengwen,Walter Elizabeth A,Gaspar Daryl Kevin S,Obodozie-Ofoegbu Obiageri O,Frei Christopher R]Int J Med Sci,2019 Jun 10;16(7):1018-1022. PMID: 31341415
5: Initial experience of bedaquiline implementation under the National TB Programme at NITRD, Delhi, India.
[Sarin Rohit,Singla Neeta,Vohra Vikram,Singla Rupak,Puri M M,Munjal Sushil,Khalid U K,Myneedu V P,Kumar Verma Ajoy,Mathuria K K]Indian J Tuberc,2019 Jan;66(1):209-213. PMID: 30878071
6: Telavancin (Vibativ). A vancomycin derivative, no more effective but more toxic.
Prescrire Int,2015 Nov;24(165):257-9. PMID: 26688891
7: Synthetic investigational new drugs for the treatment of tuberculosis.
[Kwon Yong-Soo,Koh Won-Jung]Expert Opin Investig Drugs,2016;25(2):183-93. PMID: 26576631
8: Management of drug-resistant TB in patients with HIV co-infection.
[Meintjes Graeme]J Int AIDS Soc,2014 Nov 2;17(4 Suppl 3):19508. PMID: 25394017
9: Lack of an effect of standard and supratherapeutic doses of linezolid on QTc interval prolongation.
[Damle Bharat,Labadie Robert R,Cuozzo Cheryl,Alvey Christine,Choo Heng Wee,Riley Steve,Kirby Deborah]Antimicrob Agents Chemother,2011 Sep;55(9):4302-7. PMID: 21709083
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.