Search for drugs:
Typing the drug name to query
EPLERENONE
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- There was no significant change in average heart rate among patients treated with eplerenone in the combined clinical studies. No consistent effects of eplerenone on heart rate, QRS duration, or PR or QT interval were observed in 147 normal subjects evaluated for electrocardiographic changes during pharmacokinetic studies.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
4
24088
Other ADRs
4029
38377558
Odds Ratio = 1.582
Drug Property Information
ATC Code(s):
- C03DA04 - eplerenone
- C03DA - Aldosterone antagonists
- C03D - POTASSIUM-SPARING AGENTS
- C03 - DIURETICS
- C - CARDIOVASCULAR SYSTEM
Active Ingredient:EPLERENONE
Active Ingredient UNII:6995V82D0B
Drugbank ID:DB00700
PubChem Compound:443872
CTD ID: D000077545
PharmGKB:PA164749044
CAS Number:107724-20-9
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 50.0 mg/day C03DA04
Chemical Structure: 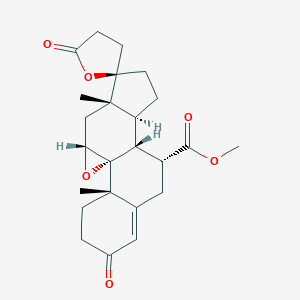

SMILE Code:
[H][C@@]12CC[C@@]3(CCC(=O)O3)[C@@]1(C)C[C@H]1O[C@@]11[C@@]2([H])[C@@H](CC2=CC(=O)CC[C@]12C)C(=O)OC
[H][C@@]12CC[C@@]3(CCC(=O)O3)[C@@]1(C)C[C@H]1O[C@@]11[C@@]2([H])[C@@H](CC2=CC(=O)CC[C@]12C)C(=O)OC
Reference
1: Sex and gender differences in cardiovascular drug therapy.
[Seeland Ute,Regitz-Zagrosek Vera]Handb Exp Pharmacol,2012;(214):211-36. PMID: 23027453
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.