Search for drugs:
Typing the drug name to query
BINIMETINIB
DIR Classification
Classification:Moderate-DIQT concern
Severity Score:2.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- DOSAGE AND ADMINISTRATION
- Dosage Modifications for Adverse Reactions
- Dose modification of MEKTOVI when administered with encorafenib is not recommended for the following adverse reactions: palmar-plantar erythrodysesthesia syndrome (PPES), non-cutaneous RAS mutation-positive malignancies, and QTc prolongation.
- ADVERSE REACTIONS
- The COLUMBUS trial [see CLINICAL STUDIES (14)] excluded patients with a history of Gilbert's syndrome, abnormal left ventricular ejection fraction, prolonged QTc (> 480 msec), uncontrolled hypertension, and history or current evidence of retinal vein occlusion. The median duration of exposure was 11.8 months for patients treated with MEKTOVI in combination with encorafenib and 6.2 months for patients treated with vemurafenib.
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- Following MEKTOVI 45 mg twice daily, no clinically meaningful QT prolongation was observed.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
8
24084
Other ADRs
6101
38375486
Odds Ratio = 2.09
Drug Property Information
ATC Code(s):
- L01EE03 - binimetinib
- L01EE0 -
- L01EE -
- L01E -
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:BINIMETINIB
Active Ingredient UNII:181R97MR71
Drugbank ID:DB11967
PubChem Compound:10288191
CTD ID:C581313
PharmGKB:PA166179867
CAS Number:606143-89-9
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 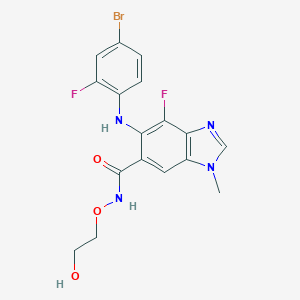

SMILE Code:
CN1C=NC2=C(F)C(NC3=CC=C(Br)C=C3F)=C(C=C12)C(=O)NOCCO
CN1C=NC2=C(F)C(NC3=CC=C(Br)C=C3F)=C(C=C12)C(=O)NOCCO
Reference
1: Cardiovascular Adverse Events Associated With BRAF and MEK Inhibitors: A Systematic Review and Meta-analysis.
[Mincu Raluca I,Mahabadi Amir A,Michel Lars,Mrotzek Simone M,Schadendorf Dirk,Rassaf Tienush,Totzeck Matthias]JAMA Netw Open,2019 Aug 2;2(8):e198890. PMID: 31397860
2: Tolerability of BRAF/MEK inhibitor combinations: adverse event evaluation and management.
[Heinzerling Lucie,Eigentler Thomas K,Fluck Michael,Hassel Jessica C,Heller-Schenck Daniela,Leipe Jan,Pauschinger Matthias,Vogel Arndt,Zimmer Lisa,Gutzmer Ralf]ESMO Open,2019 May 23;4(3):e000491. PMID: 31231568
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.