Search for drugs:
Typing the drug name to query
ALBUTEROL SULFATE
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS
- Cardiovascular Effects
- Albuterol Sulfate Syrup, like all other beta-adrenergic agonists, can produce a clinically significant cardiovascular effect in some patients as measured by pulse rate, blood pressure, and/or symptoms. Although such effects are uncommon after administration of albuterol sulfate syrup at recommended doses, if they occur, the drug may need to be discontinued. In addition, beta-agonists have been reported to produce electrocardiogram (ECG) changes, such as flattening of the T wave, prolongation of the QTc interval, and ST segment depression. The clinical significance of these findings is unknown.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
39
24053
Other ADRs
121030
38260557
Odds Ratio = 0.513
Drug Property Information
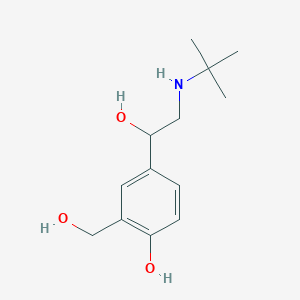
SMILE Code:
CC(C)(C)NCC(O)C1=CC(CO)=C(O)C=C1
CC(C)(C)NCC(O)C1=CC(CO)=C(O)C=C1
Reference
1: Repeat dosing of albuterol via metered-dose inhaler in infants with acute obstructive airway disease: a randomized controlled safety trial.
[Kaashmiri Mohammed,Shepard Julie,Goodman Brad,Lincourt William R,Trivedi Roopa,Ellsworth Anna,Davis Angela M]Pediatr Emerg Care,2010 Mar;26(3):197-202. PMID: 20179658
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.