Search for drugs:
Typing the drug name to query
ADENOSINE INJECTION
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS SECTION
- The following adverse events have been reported from marketing experience with adenosine. Because these events are reported voluntarily from a population of uncertain size, are associated with concomitant diseases and multiple drug therapies and surgical procedures, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Decisions to include these events in labeling are typically based on one or more of the following factors: (1) seriousness of the event, (2) frequency of the reporting, (3) strength of causal connection to the drug, or a combination of these factors.
- [Cardiovascular]
- Prolonged asystole, ventricular tachycardia, ventricular fibrillation, transient increase in blood pressure, bradycardia, atrial fibrillation, and Torsade de Pointes
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
1724
38379863
Odds Ratio = 0.0
Drug Property Information
ATC Code(s):
- C01EB10 - adenosine injection
- C01EB - Other cardiac preparations
- C01E - OTHER CARDIAC PREPARATIONS
- C01 - CARDIAC THERAPY
- C - CARDIOVASCULAR SYSTEM
Active Ingredient:ADENOSINE
Active Ingredient UNII:K72T3FS567
Drugbank ID:DB00640
PubChem Compound:60961
CTD ID:D000241
PharmGKB:PA448049
CAS Number:58-61-7
Dosage Form(s):injection, solution
Route(s) Of Administrator:intravenous
Daily Dose:
- 15.0 mg/day C01EB10
Chemical Structure: 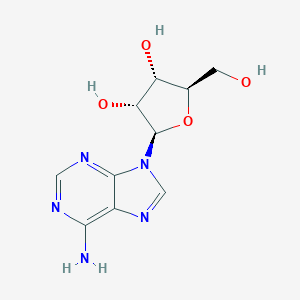

SMILE Code:
NC1=C2N=CN([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)C2=NC=N1
NC1=C2N=CN([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)C2=NC=N1
Reference
1: Provocation of sudden heart rate oscillation with adenosine exposes abnormal QT responses in patients with long QT syndrome: a bedside test for diagnosing long QT syndrome.
[Viskin Sami,Rosso Raphael,Rogowski Ori,Belhassen Bernard,Levitas Aviva,Wagshal Abraham,Katz Amos,Fourey Dana,Zeltser David,Oliva Antonio,Pollevick Guido D,Antzelevitch Charles,Rozovski Uri]Eur Heart J,2006 Feb;27(4):469-75. PMID: 16105845
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.