Search for drugs:
Typing the drug name to query
TRAZODONE HYDROCHLORIDE
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Cardiac Arrhythmias
- Clinical studies indicate that trazodone hydrochloride may be arrhythmogenic in patients with preexisting cardiac disease. Arrhythmias identified include isolated PVCs, ventricular couplets, tachycardia with syncope, and torsade de pointes. Postmarketing events, including torsade de pointes have been reported at doses of 100 mg or less with the immediate-release form of trazodone. Trazodone should also be avoided in patients with a history of cardiac arrhythmias, as well as other circumstances that may increase the risk of the occurrence of torsade de pointes and/or sudden death, including symptomatic bradycardia, hypokalemia or hypomagnesemia, and the presence of congenital prolongation of the QT interval. Trazodone is not recommended for use during the initial recovery phase of myocardial infarction. Caution should be used when administering trazodone to patients with cardiac disease and such patients should be closely monitored, since antidepressant drugs (including trazodone) may cause cardiac arrhythmias [see Adverse Reactions (6.2)].
- Trazodone prolongs the QT/QTc interval. The use of trazodone should be avoided in patients with known QT prolongation or in combination with other drugs that are inhibitors of CYP3A4 (e.g., itraconazole, clarithromycin, voriconazole), or known to prolong QT interval including Class 1A antiarrhythmics (e.g., quinidine, procainamide) or Class 3 antiarrhythmics (e.g., amiodarone, sotalol), certain antipsychotic medications (e.g., ziprasidone, chlorpromazine, thioridazine), and certain antibiotics (e.g., gatifloxacin). Concomitant administration of drugs may increase the risk of cardiac arrhythmia [see Drug Interactions (7.1)].
- DRUG INTERACTIONS
- Patients should be counseled that trazodone may enhance the response to alcohol, barbiturates, and other CNS depressants.
- Examples:
- alcohol, barbiturates
- QT Interval Prolongation
- Clinical Impact:
- Concomitant use of drugs that prolong the QT interval may add to the QT effects of trazodone and increase the risk of cardiac arrhythmia.
- Intervention:
- Avoid the use of trazodone in combination with other drugs known to prolong QTc [see Warnings and Precautions (5.3)] .
- Examples:
- Class 1A antiarrhythmics: quinidine, procainamide, disopyramide; Class 3 antiarrhythmics: amiodarone, sotalol;
- Antipsychotics: ziprasidone, chlorpromazine, thioridazine; Antibiotics: gatifloxacin
- OVERDOSAGE
- The most severe reactions reported to have occurred with overdose of trazodone alone have been priapism, respiratory arrest, seizures, and ECG changes, including QT prolongation. The reactions reported most frequently have been drowsiness and vomiting. Overdosage may cause an increase in incidence or severity of any of the reported adverse reactions.
- ADVERSE REACTIONS
- Cardiac disorders: cardiospasm, congestive heart failure, conduction block, orthostatic hypotension and syncope, palpitations, bradycardia, atrial fibrillation, myocardial infarction, cardiac arrest, arrhythmia, ventricular ectopic activity, including ventricular tachycardia and QT prolongation. Prolonged QT interval, torsade de pointes, and ventricular tachycardia have been reported at doses of 100 mg per day or less [see Warnings and Precautions (5.3)].
- MEDICATION GUIDE
- Before you take trazodone hydrochloride tablets tell your healthcare provider about all of your medical conditions, including if you:
- have heart problems, including QT prolongation or a family history of it
- See “What is the most important information I should know about trazodone hydrochloride tablets?”
- Serotonin syndrome. Symptoms of serotonin syndrome include: agitation, hallucinations, problems with coordination, fast heartbeat, tight muscles, trouble walking, sweating, fever, nausea, vomiting, and diarrhea.
- Irregular or fast heartbeat or faint (QT prolongation)
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
79
24013
Other ADRs
11708
38369879
Odds Ratio = 10.782
Drug Property Information
ATC Code(s):
- N06AX05 - trazodone hydrochloride
- N06AX - Other antidepressants
- N06A - ANTIDEPRESSANTS
- N06 - PSYCHOANALEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:TRAZODONE HYDROCHLORIDE
Active Ingredient UNII:6E8ZO8LRNM
Drugbank ID:DB00656
PubChem Compound:5533
CTD ID:D014196
PharmGKB:PA451744
CAS Number:19794-93-5
Dosage Form(s):tablet; tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 300.0 mg/day N06AX05
Chemical Structure: 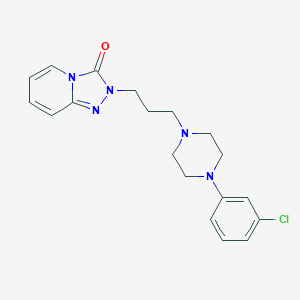

SMILE Code:
ClC1=CC=CC(=C1)N1CCN(CCCN2N=C3C=CC=CN3C2=O)CC1
ClC1=CC=CC(=C1)N1CCN(CCCN2N=C3C=CC=CN3C2=O)CC1
Reference
1: Effect of 3 Single Doses of Trazodone on QTc Interval in Healthy Subjects.
[Tellone Valeria,Rosignoli Maria Teresa,Picollo Rossella,Dragone Patrizia,Del Vecchio Alessandra,Comandini Alessandro,Radicioni Milko,Leuratti Chiara,Calisti Fabrizio]J Clin Pharmacol,2020 Nov;60(11):1483-1495. PMID: 32488885
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.