Search for drugs:
Typing the drug name to query
LOPERAMIDE HYDROCHLORIDE
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS
- Cardiac Adverse Reactions, Including Torsades de Pointes and Sudden Death
- Cases of prolongation of the QT/QTc interval, Torsades de Pointes, other ventricular arrhythmias, cardiac arrest, some resulting in death, have been reported in adults with use of higher than recommended doses per day of loperamide hydrochloride. Cases include patients who were abusing or misusing loperamide hydrochloride (see OVERDOSAGE and DRUG ABUSE AND DEPENDENCE). Cases of syncope and ventricular tachycardia have been reported in adult patients receiving the recommended dosage of loperamide hydrochloride capsules. Some of these patients were taking other drugs or had other risk factors that may have increased their risk of cardiac adverse reactions. Additionally, postmarketing cases of cardiac arrest, syncope, and respiratory depression have been reported in pediatric patients less than 2 years of age.
- Loperamide hydrochloride is contraindicated in pediatric patients less than 2 years of age due to the risks of respiratory depression and serious cardiac adverse reactions. Avoid loperamide hydrochloride dosages higher than recommended in adults and pediatric patients 2 years of age and older due to the risk of serious cardiac adverse reactions (see DOSAGE AND ADMINISTRATION, OVERDOSAGE).
- Avoid loperamide hydrochloride in:
- combination with others drugs or herbal products that are known to prolong the QT interval, including Class 1A (e.g., quinidine, procainamide) or Class III (e.g., amiodarone, sotalol) antiarrhythmics, antipsychotics (e.g., chlorpromazine, haloperidol, thioridazine, ziprasidone), antibiotics (e.g., moxifloxacin), or any other drug known to prolong the QT interval (e.g., pentamidine, levomethadyl acetate, methadone)
- patients with risk factors for QT prolongation, including patients with congenital long QT syndrome, with a history of cardiac arrhythmias or other cardiac conditions, elderly patients and those with electrolyte abnormalities.
- PRECAUTIONS
- Geriatric Use
- In general, elderly patients may be more susceptible to drug-associated effects on the QT interval. Avoid loperamide hydrochloride in elderly patients taking drugs that can result in prolongation of the QT interval (for example, Class IA or III antiarrhythmics) or in patients with risk factors for Torsades de Pointes (see WARNINGS).
- Advise patients:
- to tell their healthcare provider about all the medications they are taking, including prescription and over-the-counter medications, vitamins and herbal supplements, especially if they take Class 1A (e.g., quinidine, procainamide) or Class III (e.g., amiodarone, sotalol) antiarrhythmics, antipsychotics (e.g., chlorpromazine, haloperidol, thioridazine, ziprasidone), antibiotics (e.g., moxifloxacin), or any other drug known to prolong the QT interval (e.g., pentamidine, levomethadyl acetate, methadone).
- OVERDOSAGE
- Symptoms
- Cases of overdosage with loperamide hydrochloride (chronic ingestion of doses ranging from 70 mg to 1600 mg daily; 4 to 100 times the recommended dose) have resulted in life-threatening cardiac adverse reactions, including QT/QTc and QRS interval prolongation, Torsades de Pointes, Brugada syndrome and other ventricular arrhythmias, syncope, cardiac arrest, and death. Cases include patients who were abusing (using supratherapeutic doses in place of opioids to induce euphoria) or misusing (taking higher than recommended doses to control diarrhea or to prevent opioid withdrawal) loperamide. The following are representative cases that included cardiac adverse reactions:
- 25 year old abused loperamide and presented to the hospital on multiple occasions with symptoms of syncope, nausea, vomiting, bradycardia, hypotensive shock. The patient also experienced ventricular tachycardia, a prolonged QTc of 527 ms and QRS interval of 170 ms, frequent premature ventricular contractions, and subsequent cardiac arrest and death (elevated loperamide blood concentration of 32 ng/ml).
- 54 year old misused loperamide hydrochloride (up to 144 mg per day) as a self-treatment for chronic diarrhea for over 2 years. Signs of cardiac toxicity included syncope, prolonged QT of 500 ms sinus arrest with junctional escape rhythm, and polymorphic ventricular tachycardia, which required cardioversion and implantable cardioverter-defibrillator (ICD) management.
- 26 year old, with prior opioid abuse, presented to the hospital with recurrent syncope and developed Torsades de Pointes requiring electrical cardioversion. An ECG revealed a sinus rhythm with a heart rate of 85 bpm and a markedly prolonged QTc interval of greater than 700 ms. The patient reported ingesting 100 to 250 mg of loperamide hydrochloride with 400 mg of cimetidine daily for several months to simulate the euphoric sensation associated with opioids.
- Management
- Consider loperamide as a possible cause of cardiac arrhythmias in patients who may have a history of opioid abuse or recent ingestion of unknown drugs and in the differential diagnosis of unstable arrhythmias, prolonged QTc or QRS intervals, and Torsades de Pointes.
- If loperamide-induced cardiac toxicity is suspected, promptly discontinue the drug and initiate therapy to manage and prevent cardiac arrhythmias and serious outcomes.
- In many cases of loperamide overdosage, anti-arrhythmic medications (e.g., magnesium sulfate) were ineffective in resolving the arrhythmias and preventing further episodes of Torsades de Pointes. Electrical cardioversion and overdrive pacing, and isoproterenol continuous infusion were reported to manage QTc prolongation in the setting of overdose.
- DOSAGE AND ADMINISTRATION
- Elderly
- No formal pharmacokinetic studies were conducted in elderly subjects. However, there were no major differences reported in the drug disposition in elderly patients with diarrhea relative to young patients. No dose adjustment is required for the elderly.
- In general, elderly patients may be more susceptible to drug-associated effects of the QT interval. Avoid loperamide hydrochloride in elderly patients taking drugs that can result in prolongation of the QT interval (for example, Class IA or III antiarrhythmics) or in patients with risk factors for Torsades de Pointes (see WARNINGS).
- ADVERSE REACTIONS
- Postmarketing Experience
- Cardiac disorders
- QT/QTc interval prolongation, Torsades de Pointes, other ventricular arrhythmias, cardiac arrest, syncope, and death (see WARNINGS, OVERDOSAGE).
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
371
23721
Other ADRs
27018
38354569
Odds Ratio = 22.203
Drug Property Information
ATC Code(s):
- A07DA03 - loperamide hydrochloride
- A07DA - Antipropulsives
- A07D - ANTIPROPULSIVES
- A07 - "ANTIDIARRHEALS, INTESTINAL ANTIINFLAMMATORY/ANTIINFECTIVE "
- A - ALIMENTARY TRACT AND METABOLISM
Active Ingredient:LOPERAMIDE HYDROCHLORIDE
Active Ingredient UNII:77TI35393C
Drugbank ID:DB00836
PubChem Compound:3955
CTD ID:D008139
PharmGKB:PA450262
CAS Number:53179-11-6
Dosage Form(s):capsule
Route(s) Of Administrator:oral
Daily Dose:
- 10.0 mg/day A07DA03
Chemical Structure: 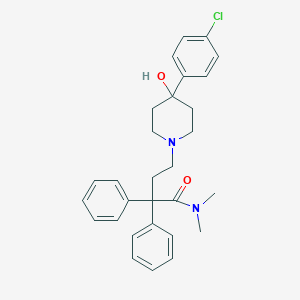

SMILE Code:
CN(C)C(=O)C(CCN1CCC(O)(CC1)C1=CC=C(Cl)C=C1)(C1=CC=CC=C1)C1=CC=CC=C1
CN(C)C(=O)C(CCN1CCC(O)(CC1)C1=CC=C(Cl)C=C1)(C1=CC=CC=C1)C1=CC=CC=C1
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.