Search for drugs:
Typing the drug name to query
GEMIFLOXACIN MESYLATE
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS
- QT Effects: Fluoroquinolones may prolong the QT interval in some patients. FACTIVE should be avoided in patients with a history of prolongation of the QTc interval, patients with uncorrected electrolyte disorders (hypokalemia or hypomagnesemia), and patients receiving Class IA (e.g., quinidine, procainamide) or Class III (e.g., amiodarone, sotalol) antiarrhythmic agents.
- Pharmacokinetic studies between gemifloxacin and drugs that prolong the QTc interval such as erythromycin, antipsychotics, and tricyclic antidepressants have not been performed. FACTIVE should be used with caution when given concurrently with these drugs, as well as in patients with ongoing proarrhythmic conditions, such as clinically significant bradycardia or acute myocardial ischemia. No cardiovascular morbidity or mortality attributable to QTc prolongation occurred with FACTIVE treatment in over 8119 patients, including 707 patients concurrently receiving drugs known to prolong the QTc interval and 7 patients with hypokalemia.
- The likelihood of QTc prolongation may increase with increasing dose of the drug; therefore, the recommended dose should not be exceeded especially in patients with renal or hepatic impairment where the Cmax and AUC are slightly higher. QTc prolongation may lead to an increased risk for ventricular arrhythmias including torsades de pointes. The maximal change in the QTc interval occurs approximately 5-10 hours following oral administration of gemifloxacin.
- PRECAUTIONS
- Inform patients of the following serious adverse reactions that have been associated with FACTIVE or other fluoroquinolone use:
- Prolongation of the QT interval: inform patients of the following:that FACTIVE may cause changes in the electrocardiogram (QTc interval prolongation);
- that FACTIVE may cause changes in the electrocardiogram (QTc interval prolongation);
- that FACTIVE should be avoided in patients receiving Class IA (e.g., quinidine, procainamide) or Class III (e.g., amiodarone, sotalol) antiarrhythmic agents;
- that FACTIVE should be used with caution in patients receiving drugs that affect the QTc interval such as cisapride, erythromycin, antipsychotics, and tricyclic antidepressants;
- to inform their physician of any personal or family history of QTc prolongation or proarrhythmic conditions such as hypokalemia, bradycardia, or recent myocardial ischemia;
- to contact their physician if they experience palpitations or fainting spells while taking FACTIVE;
- [Geriatric Use:]
- Elderly patients may be more susceptible to drug-associated effects on the QT interval. Therefore, FACTIVE should be avoided in patients taking drugs that can result in prolongation of the QT interval (e.g., Class IA or Class III antiarrhythmics) or in patients with risk factors for torsades de pointes (e.g., known QT prolongation, uncorrected hypokalemia).
- ADVERSE REACTIONS
- Post-Marketing Adverse Reactions
- The following are additional adverse reactions reported during the post-marketing use of FACTIVE. Since these reactions are reported voluntarily from a population of uncertain size, it is impossible to reliably estimate their frequency or establish a causal relationship to FACTIVE exposure:
- peripheral neuropathy that may be irreversible;
- anaphylactic reaction, erythema multiforme, skin exfoliation, facial swelling;
- exacerbation of myasthenia gravis;
- hemorrhage, increased international normalized ratio (INR), retinal hemorrhage;
- peripheral edema;
- renal failure;
- prolonged QT, supraventricular tachycardia, syncope, transient ischemic attack;
- photosensitivity/phototoxicity reaction (See PRECAUTIONS.);
- antibiotic-associated colitis;
- tendon rupture.
- MEDICATION GUIDE
- What are the possible side effects of FACTIVE?
- Serious heart rhythm changes (QTc prolongation and torsades de pointes)
- Tell your healthcare provider right away if you have a change in your heartbeat (a fast or irregular heartbeat), or if you faint. FACTIVE may cause a rare heart problem known as prolongation of the QT interval. This condition can cause an abnormal heartbeat and can be very dangerous. The chances of this happening are higher in people:
- who are elderly
- with a family history of prolonged QT interval
- with low blood potassium (hypokalemia)
- who take certain medicines to control heart rhythm (antiarrhythmics).
- ANIMAL PHARMACOLOGY
- Quinolones have been associated with prolongation of the electrocardiographic QT interval in dogs. Gemifloxacin produced no effect on the QT interval in dogs dosed orally to provide about 4 times human therapeutic plasma concentrations at Cmax, and transient prolongation after intravenous administration at more than 4 times human plasma levels at Cmax. Gemifloxacin exhibited weak activity in the cardiac IKr (hERG) channel inhibition assay, having an IC50 of approximately 270 渭M.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
1
24091
Other ADRs
2493
38379094
Odds Ratio = 0.64
Drug Property Information
ATC Code(s):
- J01MA15 - gemifloxacin mesylate
- J01MA - Fluoroquinolones
- J01M - QUINOLONE ANTIBACTERIALS
- J01 - ANTIBACTERIALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:GEMIFLOXACIN MESYLATE
Active Ingredient UNII:X4S9F8RL01
Drugbank ID:DB01155
PubChem Compound:9571107
CTD ID: D000077735
PharmGKB:PA10088
CAS Number:175463-14-6
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 320.0 mg/day J01MA15
Chemical Structure: 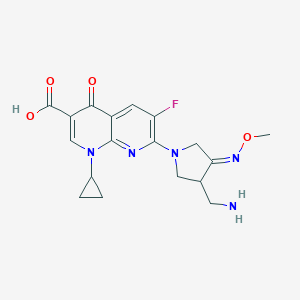

SMILE Code:
CO\N=C1/CN(CC1CN)C1=NC2=C(C=C1F)C(=O)C(=CN2C1CC1)C(O)=O
CO\N=C1/CN(CC1CN)C1=NC2=C(C=C1F)C(=O)C(=CN2C1CC1)C(O)=O
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.