Search for drugs:
Typing the drug name to query
DOLUTEGRAVIR SODIUM
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Effects on Electrocardiogram
- In a randomized, placebo-controlled, cross-over trial, 42 healthy subjects received single-dose oral administrations of placebo, dolutegravir 250-mg suspension (exposures approximately 3–fold of the 50-mg once-daily dose at steady state), and moxifloxacin 400 mg (active control) in random sequence. After baseline and placebo adjustment, the maximum mean QTc change based on Fridericia correction method (QTcF) for dolutegravir was 2.4 msec (1-sided 95% upper CI: 4.9 msec). TIVICAY did not prolong the QTc interval over 24 hours postdose.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
2
24090
Other ADRs
15889
38365698
Odds Ratio = 0.201
Drug Property Information
ATC Code(s):
- J05AJ03 - dolutegravir sodium
- J05AJ0 -
- J05AJ -
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
- J05AR21 - dolutegravir sodium
- J05AR - "Antivirals for treatment of HIV infections, combinations"
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
- J05AR25 - dolutegravir sodium
- J05AR - "Antivirals for treatment of HIV infections, combinations"
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
- J05AR13 - dolutegravir sodium
- J05AR - "Antivirals for treatment of HIV infections, combinations"
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
- J05AR27 - dolutegravir sodium
- J05AR - "Antivirals for treatment of HIV infections, combinations"
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:DOLUTEGRAVIR SODIUM
Active Ingredient UNII:1Q1V9V5WYQ
Drugbank ID:DB08930
PubChem Compound:54726191
CTD ID:C562325
PharmGKB:PA166114961
CAS Number:1051375-16-6
Dosage Form(s):tablet, film coated; tablet, for suspension
Route(s) Of Administrator:oral
Daily Dose:
- 50.0 mg/day J05AJ03
Chemical Structure: 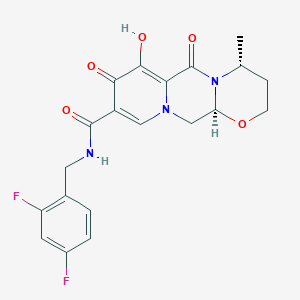

SMILE Code:
[H][C@]12CN3C=C(C(=O)NCC4=CC=C(F)C=C4F)C(=O)C(O)=C3C(=O)N1[C@H](C)CCO2
[H][C@]12CN3C=C(C(=O)NCC4=CC=C(F)C=C4F)C(=O)C(O)=C3C(=O)N1[C@H](C)CCO2
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.