Search for drugs:
Typing the drug name to query
MEMANTINE HYDROCHLORIDE
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Memantine Immediate Release Clinical Trial and Post Marketing Spontaneous Reports
- The following additional adverse reactions have been identified from previous worldwide experience with memantine (immediate release) use. These adverse reactions have been chosen for inclusion because of a combination of seriousness, frequency of reporting, or potential causal connection to memantine and have not been listed elsewhere in labeling. However, because some of these adverse reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship between their occurrence and the administration of memantine. These events include:
- atrial fibrillation, atrioventricular block (including 2nd and 3rd degree block), cardiac failure, orthostatic hypotension, and torsades de pointes. Cardiac Disorders:
- electrocardiogram QT prolonged, international normalized ratio increased. Investigations:
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
57
24035
Other ADRs
23463
38358124
Odds Ratio = 3.878
Drug Property Information
ATC Code(s):
- N06DX01 - memantine hydrochloride
- N06DX0 -
- N06DX - Other anti-dementia drugs
- N06D - ANTI-DEMENTIA DRUGS
- N06 - PSYCHOANALEPTICS
- N - NERVOUS SYSTEM
- N06DA52 - memantine hydrochloride
- N06DA - Anticholinesterases
- N06D - ANTI-DEMENTIA DRUGS
- N06 - PSYCHOANALEPTICS
- N - NERVOUS SYSTEM
- N06DA53 - memantine hydrochloride
- N06DA - Anticholinesterases
- N06D - ANTI-DEMENTIA DRUGS
- N06 - PSYCHOANALEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:MEMANTINE HYDROCHLORIDE
Active Ingredient UNII:JY0WD0UA60
Drugbank ID:DB01043
PubChem Compound:4054
CTD ID:D008559
PharmGKB:PA10364
CAS Number:19982-08-2
Dosage Form(s):capsule, extended release
Route(s) Of Administrator:oral
Daily Dose:
- 20.0 mg/day N06DX01
Chemical Structure: 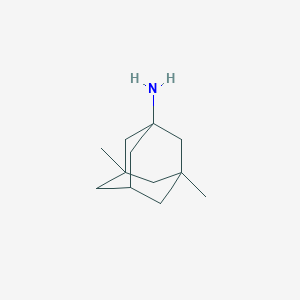

SMILE Code:
CC12CC3CC(C)(C1)CC(N)(C3)C2
CC12CC3CC(C)(C1)CC(N)(C3)C2
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.