Search for drugs:
Typing the drug name to query
NILOTINIB
DIR Classification
Classification:Most-DIQT concern
Severity Score:5.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- BOXED WARNING
- WARNING: QT PROLONGATION AND SUDDEN DEATHS
- Tasigna prolongs the QT interval. Prior to Tasigna administration and periodically, monitor for hypokalemia or hypomagnesemia and correct deficiencies [see Warnings and Precautions (5.2)]. Obtain ECGs to monitor the QTc at baseline, seven days after initiation, and periodically thereafter, and following any dose adjustments [see Warnings and Precautions (5.2, 5.3, 5.7, 5.12)].
- Sudden deaths have been reported in patients receiving Tasigna [see Warnings and Precautions (5.3)]. Do not administer Tasigna to patients with hypokalemia, hypomagnesemia, or long QT syndrome [see Contraindications (4), Warnings and Precautions (5.2)].
- Avoid use of concomitant drugs known to prolong the QT interval and strong CYP3A4 inhibitors [see Drug Interactions (7.1, 7.2)].
- Avoid food 2 hours before and 1 hour after taking the dose [see Dosage and Administration (2.1)].
- WARNINGS AND PRECAUTIONS
- QT Prolongation
- Tasigna has been shown to prolong cardiac ventricular repolarization as measured by the QT interval on the surface electrocardiogram (ECG) in a concentration-dependent manner [see Adverse Reactions (6.1), Clinical Pharmacology (12.2)]. Prolongation of the QT interval can result in a type of ventricular tachycardia called torsade de pointes, which may result in syncope, seizure, and/or death. Electrocardiograms should be performed at baseline, 7 days after initiation of Tasigna, and periodically as clinically indicated and following dose adjustments [see Warnings and Precautions (5.12)].
- Tasigna should not be used in patients who have hypokalemia, hypomagnesemia, or long QT syndrome. Before initiating Tasigna and periodically, test electrolyte, calcium, and magnesium blood levels. Hypokalemia or hypomagnesemia must be corrected prior to initiating Tasigna and these electrolytes should be monitored periodically during therapy [see Warnings and Precautions (5.12)].
- Significant prolongation of the QT interval may occur when Tasigna is inappropriately taken with food and/or strong CYP3A4 inhibitors and/or medicinal products with a known potential to prolong QT. Therefore, coadministration with food must be avoided and concomitant use with strong CYP3A4 inhibitors and/or medicinal products with a known potential to prolong QT should be avoided [see Dosage and Administration (2.1), Drug Interactions (7.1, 7.2)]. The presence of hypokalemia and hypomagnesemia may further prolong the QT interval [see Warnings and Precautions (5.7, 5.12)].
- DOSAGE AND ADMINISTRATION
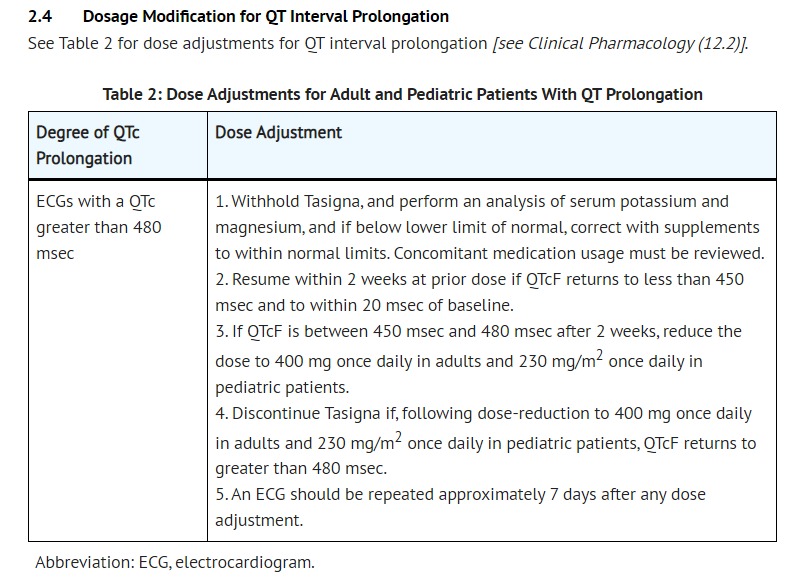
- [Dosage Modification With Concomitant Strong CYP3A4 Inhibitors]
- Avoid the concomitant use of strong CYP3A4 inhibitors. Should treatment with any of these agents be required, interrupt therapy with Tasigna. If patients must be coadministered a strong CYP3A4 inhibitor, reduce dosage to 300 mg once daily in patients with resistant or intolerant Ph+ CML or to 200 mg once daily in patients with newly diagnosed Ph+ CML-CP. However, there are no clinical data with this dose adjustment in patients receiving strong CYP3A4 inhibitors. If the strong inhibitor is discontinued, allow a washout period before adjusting Tasigna dose upward to the indicated dose. For patients who cannot avoid use of strong CYP3A4 inhibitors, monitor closely for prolongation of the QT interval [see Boxed Warning, Warnings and Precautions (5.2), Drug Interactions (7.1, 7.2), Clinical Pharmacology (12.3)].
- ADVERSE REACTIONS
- Clinical Trials Experience
- Increase in QTcF greater than 60 msec from baseline was observed in 1 patient (0.4%) in the 300 mg twice daily treatment group. No patient had an absolute QTcF of greater than 500 msec while on study drug.
- Sudden deaths and QT prolongation were reported. The maximum mean QTcF change from baseline at steady-state was 10 msec. Increase in QTcF greater than 60 msec from baseline was observed in 4.1% of the patients and QTcF of greater than 500 msec was observed in 4 patients (less than 1%) [see Boxed Warning, Warnings and Precautions (5.2, 5.3), Clinical Pharmacology (12.2)].
- The following adverse drug reactions were reported in adult patients in the Tasigna clinical studies at the recommended doses. These adverse drug reactions are ranked under a heading of frequency, the most frequent first using the following convention: common (greater than or equal to 1% and less than 10%), uncommon (greater than or equal to 0.1% and less than 1%), and unknown frequency (single events). For laboratory abnormalities, very common events (greater than or equal to 10%), which were not included in Tables 7 and 8, are also reported. These adverse reactions are included based on clinical relevance and ranked in order of decreasing seriousness within each category, obtained from 2 clinical studies:
- Cardiac Disorders: Common: angina pectoris, arrhythmia (including atrioventricular block, cardiac flutter, extrasystoles, atrial fibrillation, tachycardia, bradycardia), palpitations, electrocardiogram QT prolonged. Uncommon: cardiac failure, myocardial infarction, coronary artery disease, cardiac murmur, coronary artery stenosis, myocardial ischemia, pericardial effusion, cyanosis. Unknown frequency: ventricular dysfunction, pericarditis, ejection fraction decrease.
- [Drugs That Prolong the QT Interval]
- Avoid coadministration of Tasigna with agents that may prolong the QT interval, such as anti-arrhythmic drugs [see Boxed Warning, Dosage and Administration (2.4), Warnings and Precautions (5.2), Drug Interactions (7.1), Clinical Pharmacology (12.2)].
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- Tasigna is associated with concentration-dependent QT prolongation. At a dose of Tasigna 400 mg twice daily given without food in healthy subjects, the maximum mean placebo-adjusted QTcF changes were 10.4 msec (90% CI: 2.85, 18.0). After a single dose of Tasigna 800 mg (two times the maximum approved recommended dosage) given with a high fat meal to healthy subjects, the maximum mean placebo-adjusted QTcF changes were) 18.0 msec (90% CI: 9.65, 25.8). Peak plasma concentrations in the QT study were 26% lower than or comparable with those observed in patients enrolled in the single-arm study [see Boxed Warning, Warnings and Precautions (5.2), Adverse Reactions (6.1)].
- PATIENT COUNSELING INFORMATION
- QT Prolongation
- Advise patients that Tasigna can cause possibly life-threatening, abnormal heart beat. Advise patients to seek immediate medical attention if symptoms of abnormal heart beat occur, such as feeling light-headed, faint or experiencing an irregular heartbeat [see Warnings and Precautions (5.2)].
- MEDICATION GUIDE
- Tasigna can cause a possible life-threatening heart problem called QTc prolongation. QTc prolongation causes an irregular heartbeat, which may lead to sudden death.
- You may lower your chances for having QTc prolongation with Tasigna if you:
- Take Tasigna on an empty stomach:
- Avoid eating food for at least 2 hours before the dose is taken, and
- Avoid eating food for at least 1 hour after the dose is taken.
- Avoid grapefruit, grapefruit juice, and any supplement containing grapefruit extract during treatment with Tasigna. Food and grapefruit products increase the amount of Tasigna in your body.
- Avoid taking other medicines or supplements with Tasigna that can also cause QTc prolongation.
- Tasigna can interact with many medicines and supplements and increase your chance for serious and life-threatening side effects.
- Do not take any other medicine during treatment with Tasigna unless your healthcare provider tells you it is okay to do so.
- If you cannot swallow Tasigna capsules whole, you may open the Tasigna capsule and sprinkle the contents of each capsule in 1 teaspoon of applesauce (puréed apple). Swallow the mixture right away (within 15 minutes). For more information, see “How should I take Tasigna?
- USE IN SPECIFIC POPULATIONS
- Hepatic Impairment
- Reduce the Tasigna dosage in patients with hepatic impairment and monitor the QT interval closely in these patients [see Dosage and Administration (2.7), Clinical Pharmacology (12.3)].
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
640
23452
Other ADRs
70914
38310673
Odds Ratio = 14.744
Drug Property Information
ATC Code(s):
- L01EA03 - nilotinib
- L01EA -
- L01E -
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:NILOTINIB
Active Ingredient UNII:F41401512X
Drugbank ID:DB04868
PubChem Compound:644241
CTD ID:C498826
PharmGKB:PA165958345
CAS Number:641571-10-0
Dosage Form(s):capsule
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 

SMILE Code:
CC1=CN(C=N1)C1=CC(NC(=O)C2=CC=C(C)C(NC3=NC=CC(=N3)C3=CN=CC=C3)=C2)=CC(=C1)C(F)(F)F
CC1=CN(C=N1)C1=CC(NC(=O)C2=CC=C(C)C(NC3=NC=CC(=N3)C3=CN=CC=C3)=C2)=CC(=C1)C(F)(F)F
Reference
1: Risk of QTc prolongation among cancer patients treated with tyrosine kinase inhibitors.
[Abu Rmilah Anan A,Lin Grace,Begna Kebede H,Friedman Paul A,Herrmann Joerg]Int J Cancer,2020 Dec 1;147(11):3160-3167. PMID: 32449208
2: Cardiovascular Toxicity of Tyrosine Kinase Inhibitors Used in Chronic Myeloid Leukemia: An Analysis of the FDA Adverse Event Reporting System Database (FAERS).
[Cirmi Santa,El Abd Asmae,Letinier Louis,Navarra Michele,Salvo Francesco]Cancers (Basel),2020 Mar 30;12(4):826. PMID: 32235443
3: Cardiovascular Complications of Targeted Therapies for Chronic Myeloid Leukemia.
[Damrongwatanasuk Rongras,Fradley Michael G]Curr Treat Options Cardiovasc Med,2017 Apr;19(4):24. PMID: 28316033
4: Switching to nilotinib versus imatinib dose escalation in patients with chronic myeloid leukaemia in chronic phase with suboptimal response to imatinib (LASOR): a randomised, open-label trial.
[Cortes Jorge E,De Souza Carmino Antonio,Ayala Manuel,Lopez Jose Luis,Bullorsky Eduardo,Shah Sandip,Huang Xiaojun,Babu K Govind,Abdulkadyrov Kudrat,de Oliveira José Salvador Rodrigues,Shen Zhi-Xiang,Sacha Tomasz,Bendit Israel,Liang Zhizhou,Owugah Tina,Szczudlo Tomasz,Khanna Sadhvi,Fellague-Chebra Rafik,le Coutre Philipp D]Lancet Haematol,2016 Dec;3(12):e581-e591. PMID: 27890073
5: Time-to-Onset Analysis of Drug-Induced Long QT Syndrome Based on a Spontaneous Reporting System for Adverse Drug Events.
[Sasaoka Sayaka,Matsui Toshinobu,Hane Yuuki,Abe Junko,Ueda Natsumi,Motooka Yumi,Hatahira Haruna,Fukuda Akiho,Naganuma Misa,Hasegawa Shiori,Kinosada Yasutomi,Nakamura Mitsuhiro]PLoS One,2016 Oct 10;11(10):e0164309. PMID: 27723808
6: [Achievement of deep molecular response in an elderly chronic myeloid leukemia patient intolerant to imatinib and nilotinib].
[Kurimoto Miwa,Nagata Akihisa,Sekiguchi Naohiro,Noto Satoshi,Takezako Naoki]Rinsho Ketsueki,2015 Dec;56(12):2467-71. PMID: 26725357
7: Nilotinib (Tasigna™) in the treatment of early diffuse systemic sclerosis: an open-label, pilot clinical trial.
[Gordon Jessica K,Martyanov Viktor,Magro Cynthia,Wildman Horatio F,Wood Tammara A,Huang Wei-Ti,Crow Mary K,Whitfield Michael L,Spiera Robert F]Arthritis Res Ther,2015 Aug 18;17(1):213. PMID: 26283632
8: Liposomes ameliorate Crizotinib- and Nilotinib-induced inhibition of the cardiac IKr channel and QTc prolongation.
[Shopp George M,Helson Lawrence,Bouchard Annie,Salvail Dany,Majeed Muhammad]Anticancer Res,2014 Sep;34(9):4733-40. PMID: 25202051
9: Potential interactions between HIV drugs, ritonavir and efavirenz and anticancer drug, nilotinib--a study in primary cultures of human hepatocytes that is applicable to HIV patients with cancer.
[Pillai Venkateswaran C,Parise Robert A,Christner Susan M,Rudek Michelle A,Beumer Jan H,Venkataramanan Raman]J Clin Pharmacol,2014 Nov;54(11):1272-9. PMID: 24846165
10: Clinical cardiac safety profile of nilotinib.
[Kim Theo D,le Coutre Philipp,Schwarz Michaela,Grille Peggy,Levitin Michal,Fateh-Moghadam Suzanne,Giles Francis J,Dörken Bernd,Haverkamp Wilhelm,Köhncke Clemens]Haematologica,2012 Jun;97(6):883-9. PMID: 22271904
11: Identification of side effects associated with intolerance to BCR-ABL inhibitors in patients with chronic myeloid leukemia.
[Rios Mary Beth,Ault Patricia]Clin J Oncol Nurs,2011 Dec;15(6):660-7. PMID: 22119977
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.