Search for drugs:
Typing the drug name to query
OSIMERTINIB
DIR Classification
Classification:Moderate-DIQT concern
Severity Score:3.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QTc Interval Prolongation
- Heart rate-corrected QT (QTc) interval prolongation occurs in patients treated with TAGRISSO. Of the 1479 patients treated with TAGRISSO in clinical trials, 0.8% were found to have a QTc > 500 msec, and 3.1% of patients had an increase from baseline QTc > 60 msec [see CLINICAL PHARMACOLOGY (12.2)]. No QTc-related arrhythmias were reported.
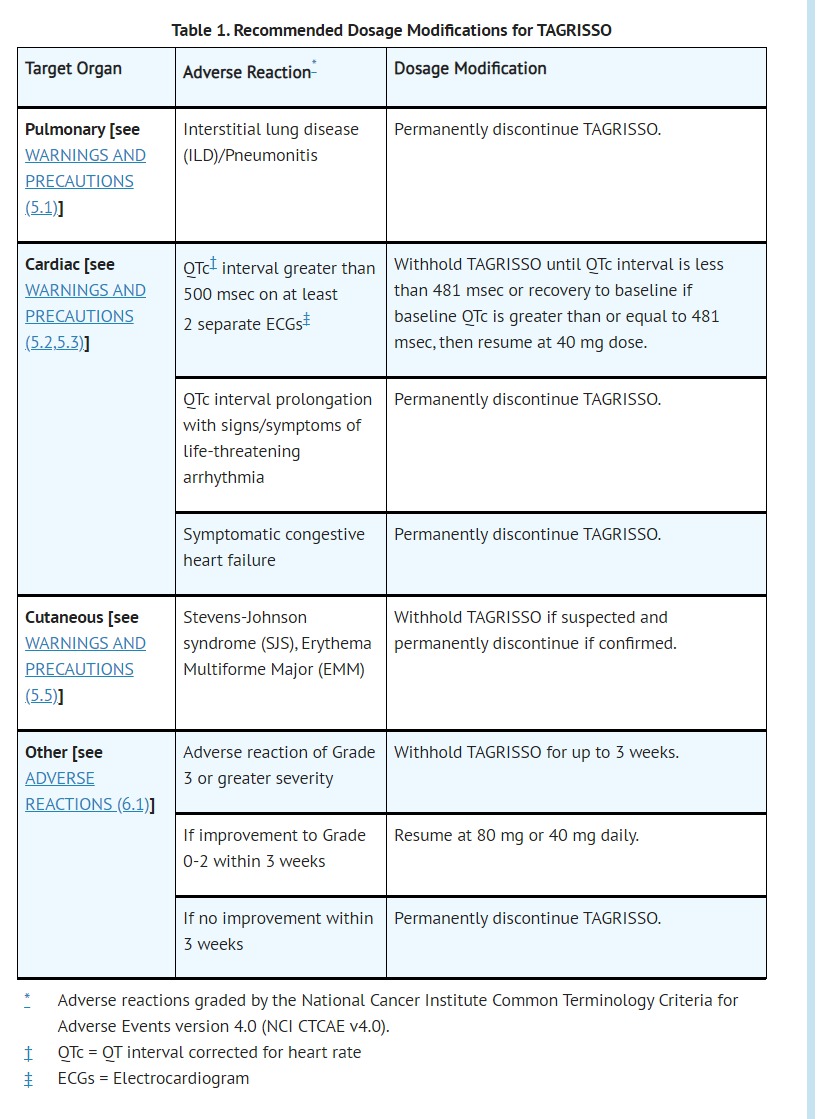
- Clinical trials of TAGRISSO did not enroll patients with baseline QTc of > 470 msec. Conduct periodic monitoring with ECGs and electrolytes in patients with congenital long QTc syndrome, congestive heart failure, electrolyte abnormalities, or those who are taking medications known to prolong the QTc interval. Permanently discontinue TAGRISSO in patients who develop QTc interval prolongation with signs/symptoms of life-threatening arrhythmia [see DOSAGE AND ADMINISTRATION (2.4)].
- DRUG INTERACTIONS
- Drugs That Prolong the QTc Interval
- The effect of co-administering medicinal products known to prolong the QTc interval with TAGRISSO is unknown. When feasible, avoid concomitant administration of drugs known to prolong the QTc interval with known risk of Torsades de pointes. If not feasible to avoid concomitant administration of such drugs, conduct periodic ECG monitoring [see WARNINGS AND PRECAUTIONS (5.2) and CLINICAL PHARMACOLOGY (12.3)].
- DOSAGE AND ADMINISTRATION
- ADVERSE REACTIONS
- Clinical Trials Experience
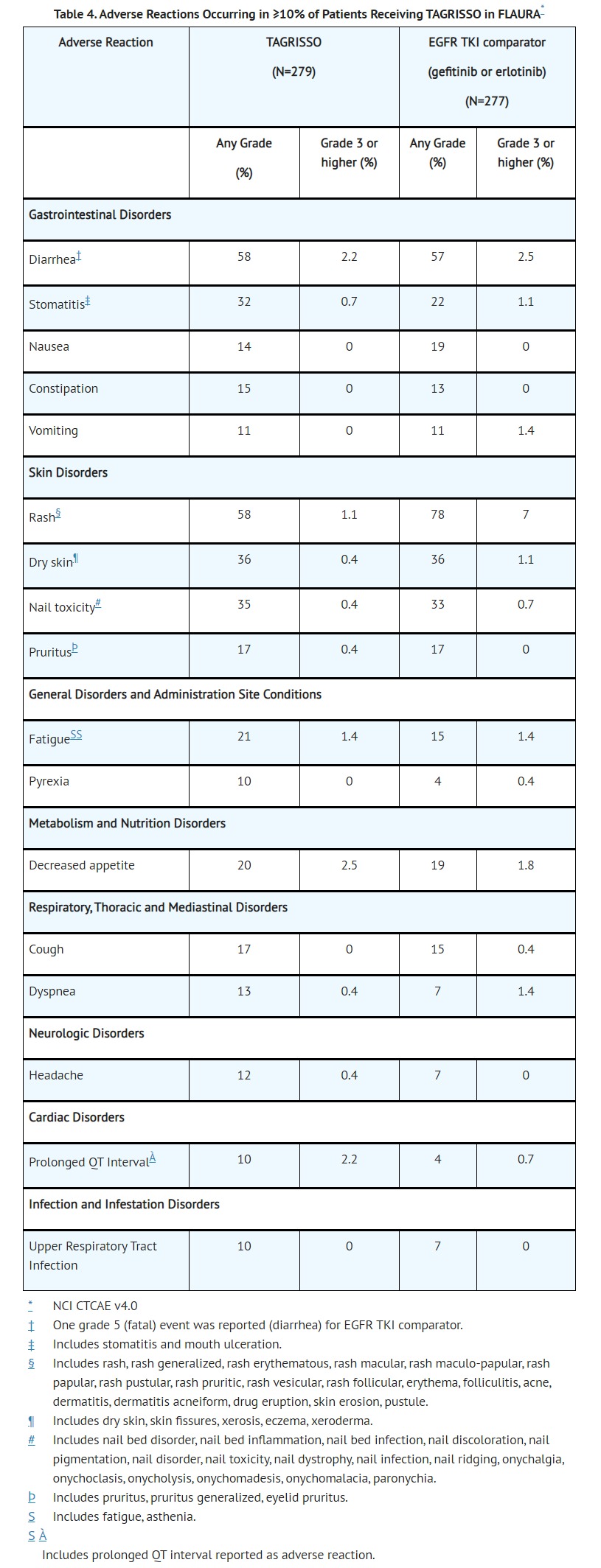
- The data described below reflect exposure to TAGRISSO (80 mg daily) in 337 patients with EGFR mutation-positive resectable NSCLC, and 558 patients with EGFR mutation-positive metastatic NSCLC in three randomized, controlled trials [ADAURA (n=337), FLAURA (n=279), and AURA3 (n=279)]. Patients with a history of interstitial lung disease, drug induced interstitial disease or radiation pneumonitis that required steroid treatment, serious arrhythmia or baseline QTc interval greater than 470 msec on electrocardiogram were excluded from enrollment in these studies.
- linically relevant adverse reactions in ADAURA in <10% of patients receiving TAGRISSO were alopecia (6%), epistaxis (6%), interstitial lung disease (3%), palmar-plantar erythrodysesthesia syndrome (1.8%), keratitis (0.6%), QTc interval prolongation (0.6%), and erythema multiform (0.3%). QTc interval prolongation represents the incidence of patients who had a QTcF prolongation >500msec.
- Serious adverse reactions were reported in 4% of patients treated with TAGRISSO; the most common serious adverse reactions (≥1%) were pneumonia (2.9%), ILD/pneumonitis (2.1%), and pulmonary embolism (1.8%). Dose reductions occurred in 2.9% of patients treated with TAGRISSO. The most frequent adverse reactions leading to dose reductions or interruptions were prolongation of the QT interval as assessed by ECG (4.3%), diarrhea (2.5%), and lymphopenia (1.1%). Adverse reactions leading to permanent discontinuation occurred in 13% of patients treated with TAGRISSO. The most frequent adverse reaction leading to discontinuation of TAGRISSO was ILD/pneumonitis (3.9%).
- Clinically relevant adverse reactions in FLAURA in <10% of patients receiving TAGRISSO were alopecia (7%), epistaxis (6%), interstitial lung disease (3.9%), palmar-plantar erythrodysesthesia syndrome (1.4%), QTc interval prolongation (1.1%), and keratitis (0.4%). QTc interval prolongation represents the incidence of patients who had a QTcF prolongation >500msec.
- Dose reductions occurred in 2.9% of patients treated with TAGRISSO. The most frequent adverse reactions leading to dose reductions or interruptions were prolongation of the QT interval as assessed by ECG (1.8%), neutropenia (1.1%), and diarrhea (1.1%). Adverse reactions resulting in permanent discontinuation of TAGRISSO occurred in 7% of patients treated with TAGRISSO. The most frequent adverse reaction leading to discontinuation of TAGRISSO was ILD/pneumonitis (3%).
- Clinically relevant adverse reactions in AURA3 in <10% of patients receiving TAGRISSO were epistaxis (5%), interstitial lung disease (3.9%), alopecia (3.6%), palmar-plantar erythrodysesthesia syndrome (1.8%), QTc interval prolongation (1.4%), keratitis (1.1%), and erythema multiform (0.7%). QTc interval prolongation represents the incidence of patients who had a QTcF prolongation >500msec.
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- The QTc interval prolongation potential of osimertinib was assessed in 210 patients who received TAGRISSO 80 mg daily in AURA2. A central tendency analysis of the QTcF data at steady-state demonstrated that the maximum mean change from baseline was 16.2 msec (upper bound of two-sided 90% confidence interval (CI) 17.6 msec). A pharmacokinetic/pharmacodynamic analysis in AURA2 suggested a concentration-dependent QTc interval prolongation of 14 msec (upper bound of two-sided 90% CI: 16 msec) at a dose of TAGRISSO 80 mg.
- PATIENT COUNSELING INFORMATION
- QTc Interval Prolongation
- Inform patients of symptoms that may be indicative of significant QTc prolongation including dizziness, lightheadedness, and syncope. Advise patients to report these symptoms and to inform their physician about the use of any heart or blood pressure medications [see WARNINGS AND PRECAUTIONS (5.2)].
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
82
24010
Other ADRs
17573
38364014
Odds Ratio = 7.456
Drug Property Information
ATC Code(s):
- L01EB04 - osimertinib
- L01EB -
- L01E -
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:OSIMERTINIB
Active Ingredient UNII:3C06JJ0Z2O
Drugbank ID:DB09330
PubChem Compound:71496458
CTD ID: C000596361
PharmGKB:PA166131626
CAS Number:1421373-65-0
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 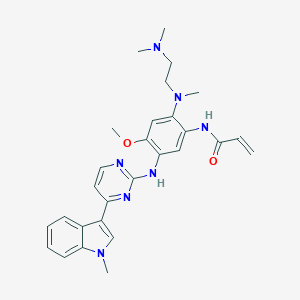

SMILE Code:
COC1=C(NC2=NC=CC(=N2)C2=CN(C)C3=C2C=CC=C3)C=C(NC(=O)C=C)C(=C1)N(C)CCN(C)C
COC1=C(NC2=NC=CC(=N2)C2=CN(C)C3=C2C=CC=C3)C=C(NC(=O)C=C)C(=C1)N(C)CCN(C)C
Reference
1: Case Report: QT Prolongation and Abortive Sudden Death Observed in an 85-Year-Old Female Patient With Advanced Lung Cancer Treated With Tyrosine Kinase Inhibitor Osimertinib.
[Kondo Moë,Kisanuki Megumi,Kokawa Yosuke,Gohara Seiichiro,Kawano Osamu,Kagiyama Shuntaro,Maruyama Toru,Odashiro Keita,Maehara Yoshihiko]Front Cardiovasc Med,2021 Mar 19;8:655808. PMID: 33816581
2: Osimertinib-induced cardiac failure with QT prolongation and torsade de pointes in a patient with advanced pulmonary adenocarcinoma.
[Ikebe Saori,Amiya Ryohei,Minami Seigo,Ihara Shoichi,Higuchi Yoshiharu,Komuta Kiyoshi]Int Cancer Conf J,2020 Oct 15;10(1):68-71. PMID: 33489705
3: Osimertinib induced cardiomyopathy: A case report.
[Shinomiya Shun,Kaira Kyoichi,Yamaguchi Ou,Ishikawa Keitaro,Kagamu Hiroshi]Medicine (Baltimore),2020 Sep 25;99(39):e22301. PMID: 32991436
4: A case of torsades de pointes induced by the third-generation EGFR-TKI, osimertinib combined with moxifloxacin.
[Bian Shuang,Tang Xiaomiao,Lei Wei]BMC Pulm Med,2020 Jun 24;20(1):181. PMID: 32580784
5: Current perspective: Osimertinib-induced QT prolongation: new drugs with new side-effects need careful patient monitoring.
[Schiefer Mart,Hendriks Lizza E L,Dinh Trang,Lalji Ulrich,Dingemans Anne-Marie C]Eur J Cancer,2018 Mar;91:92-98. PMID: 29413968
6: AC0010, an Irreversible EGFR Inhibitor Selectively Targeting Mutated EGFR and Overcoming T790M-Induced Resistance in Animal Models and Lung Cancer Patients.
[Xu Xiao,Mao Long,Xu Wanhong,Tang Wei,Zhang Xiaoying,Xi Biao,Xu Rongda,Fang Xin,Liu Jia,Fang Ce,Zhao Li,Wang Xiaobo,Jiang Ji,Hu Pei,Zhao Hongyun,Zhang Li]Mol Cancer Ther,2016 Nov;15(11):2586-2597. PMID: 27573423
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.