Search for drugs:
Typing the drug name to query
TROSPIUM CHLORIDE
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Electrophysiology
- The effect of 20 mg twice daily and up to 100 mg twice daily of an immediate-release formulation of trospium chloride on QT interval was evaluated in a single-blind, randomized, placebo and active (moxifloxacin 400 mg daily) controlled, 5-day parallel trial in 170 male and female healthy volunteer subjects aged 18 to 45 years. The QT interval was measured over a 24-hour period at steady state. Trospium chloride was not associated with an increase in individual corrected (QTcI) or Fridericia corrected (QTcF) QT interval at any time during steady state measurement, while moxifloxacin was associated with a 6.4 msec increase in QTcF.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
1
24091
Other ADRs
2054
38379533
Odds Ratio = 0.776
Drug Property Information
ATC Code(s):
- G04BD09 - trospium chloride
- G04BD - Urinary antispasmodics
- G04B - "OTHER UROLOGICALS, INCL. ANTISPASMODICS"
- G04 - UROLOGICALS
- G - GENITO URINARY SYSTEM AND SEX HORMONES
- A03DA06 - trospium chloride
- A03DA - Synthetic anticholinergic agents in combination with analgesics
- A03D - ANTISPASMODICS IN COMBINATION WITH ANALGESICS
- A03 - DRUGS FOR FUNCTIONAL GASTROINTESTINAL DISORDERS
- A - ALIMENTARY TRACT AND METABOLISM
Active Ingredient:TROSPIUM CHLORIDE
Active Ingredient UNII:1E6682427E
Drugbank ID:DB00209
PubChem Compound:5284632
CTD ID:C003330
PharmGKB:PA164748976
CAS Number:47608-32-2
Dosage Form(s):capsule, extended release
Route(s) Of Administrator:oral
Daily Dose:
- 40.0 mg/day G04BD09
Chemical Structure: 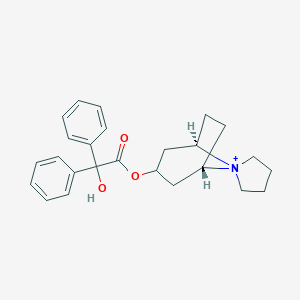

SMILE Code:
[H][C@]12CC[C@]([H])(C[C@@H](C1)OC(=O)C(O)(C1=CC=CC=C1)C1=CC=CC=C1)[N+]21CCCC1
[H][C@]12CC[C@]([H])(C[C@@H](C1)OC(=O)C(O)(C1=CC=CC=C1)C1=CC=CC=C1)[N+]21CCCC1
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.