Search for drugs:
Typing the drug name to query
IVOSIDENIB
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QTc Interval Prolongation
- Patients treated with TIBSOVO can develop QT (QTc) prolongation [see Clinical Pharmacology (12.2)] and ventricular arrhythmias. Of the 258 patients with hematological malignancies treated with TIBSOVO in the clinical trial, 9% were found to have a QTc interval greater than 500 msec and 14% of patients had an increase from baseline QTc greater than 60 msec. One patient developed ventricular fibrillation attributed to TIBSOVO. The clinical trial excluded patients with baseline QTc of ≥ 450 msec (unless the QTc ≥ 450 msec was due to a pre-existing bundle branch block) or with a history of long QT syndrome or uncontrolled or significant cardiovascular disease.
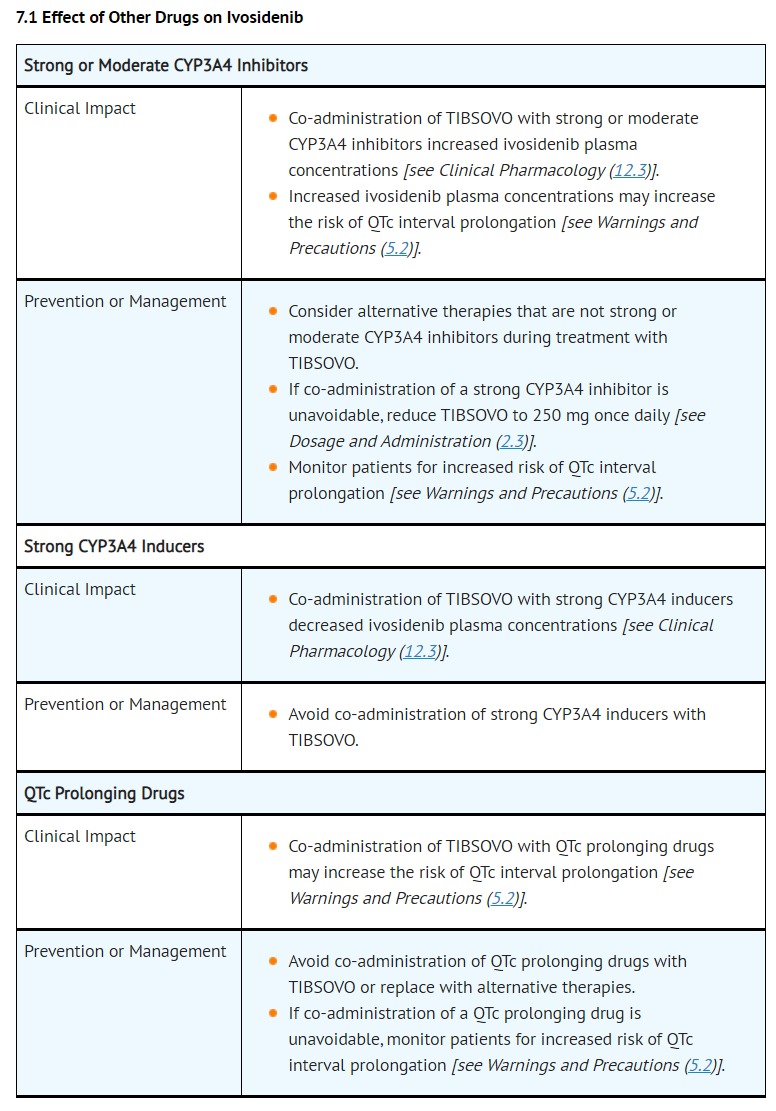
- Concomitant use of TIBSOVO with drugs known to prolong the QTc interval (e.g., anti-arrhythmic medicines, fluoroquinolones, triazole anti-fungals, 5-HT3 receptor antagonists) and CYP3A4 inhibitors may increase the risk of QTc interval prolongation [see Drug Interactions (7.1), Clinical Pharmacology (12.2)]. Conduct monitoring of electrocardiograms (ECGs) and electrolytes [see Dosage and Administration (2.3)].
- In patients with congenital long QTc syndrome, congestive heart failure, electrolyte abnormalities, or those who are taking medications known to prolong the QTc interval, more frequent monitoring may be necessary.
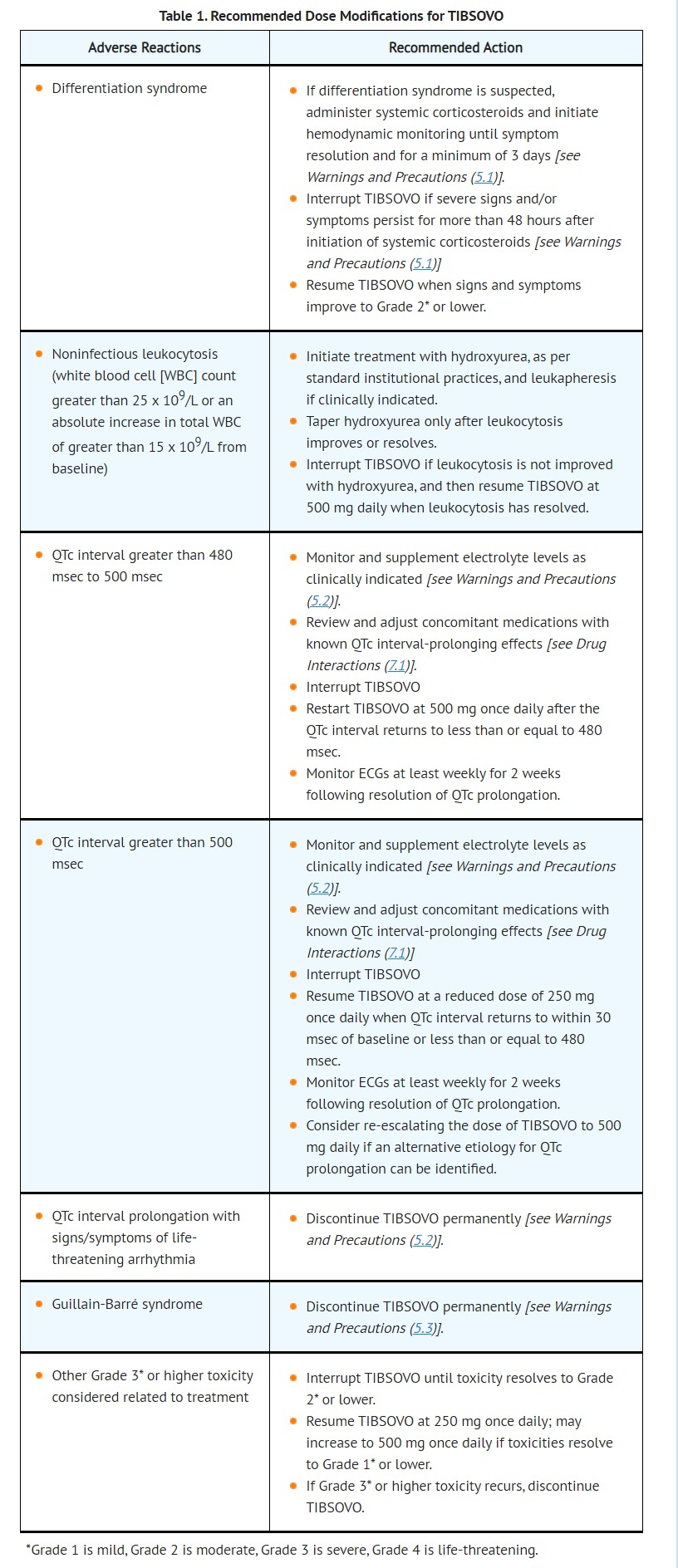
- Interrupt TIBSOVO if QTc increases to greater than 480 msec and less than 500 msec. Interrupt and reduce TIBSOVO if QTc increases to greater than 500 msec. Permanently discontinue TIBSOVO in patients who develop QTc interval prolongation with signs or symptoms of life-threatening arrhythmia [see Dosage and Administration (2.3)].
- DRUG INTERACTIONS
- DOSAGE AND ADMINISTRATION
- ADVERSE REACTIONS
- Clinical Trials Experience
- The safety of TIBSOVO as a single agent at 500 mg daily was evaluated in 213 patients with AML in Study AG120-C-001 [see Clinical Studies (14.1 and 14.2)]. The median age of TIBSOVO treated patients was 68 (range 18-87) with 68% ≥ 65 years, 51% male, 66% White, 6% Black or African American, 3% Asian, 0.5% Native Hawaiian or other Pacific Islander, 0.5% American Indian or Alaska Native, and 24% other/not provided. Among the 213 patients who received TIBSOVO, 37% were exposed for 6 months or longer and 14% were exposed for 12 months or longer. The most common adverse reactions including laboratory abnormalities in ≥ 20% of 213 patients who received TIBSOVO were hemoglobin decreased, fatigue, arthralgia, calcium decreased, sodium decreased, leukocytosis, diarrhea, magnesium decreased, edema, nausea, dyspnea, uric acid increased, potassium decreased, alkaline phosphatase increased, mucositis, aspartate aminotransferase increased, phosphatase decreased, electrocardiogram QT prolonged, rash, creatinine increased, cough, decreased appetite, myalgia, constipation, and pyrexia.
- Newly-Diagnosed AML
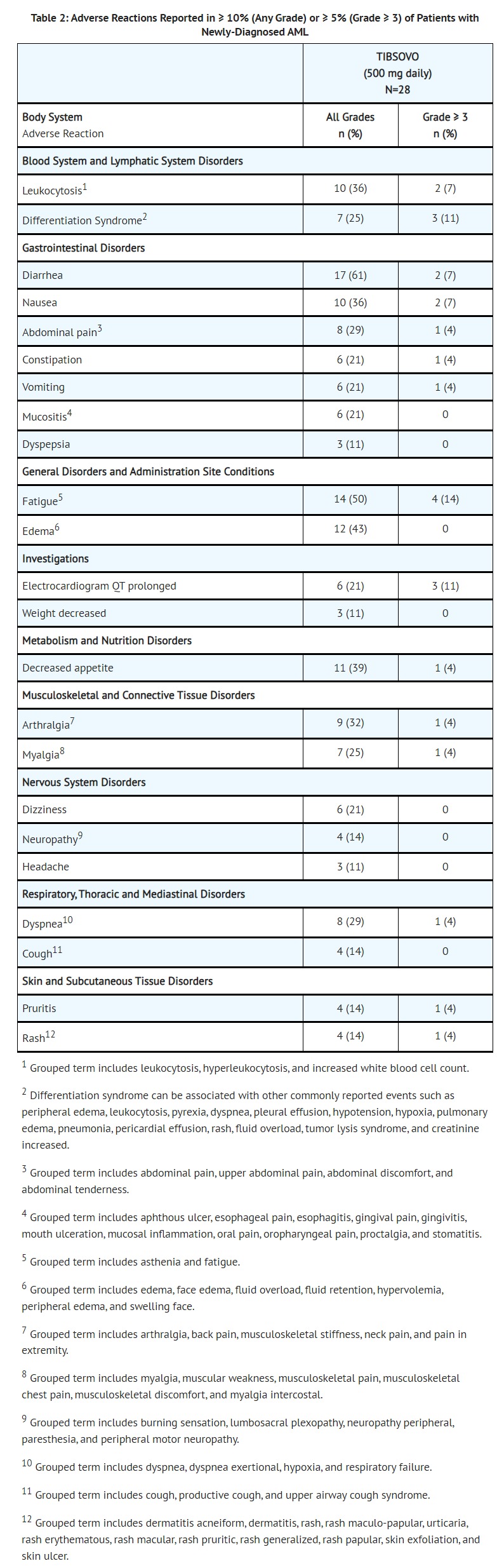
- The safety profile of single-agent TIBSOVO was studied in 28 adults with newly-diagnosed AML treated with 500 mg daily [see Clinical Studies (14.1)]. The median duration of exposure to TIBSOVO was 4.3 months (range 0.3 to 40.9 months). Ten patients (36%) were exposed to TIBSOVO for at least 6 months and 6 patients (21%) were exposed for at least 1 year.
- Common (≥ 5%) serious adverse reactions included differentiation syndrome (18%), electrocardiogram QT prolonged (7%), and fatigue (7%). There was one case of posterior reversible encephalopathy syndrome (PRES).
- Common (≥ 10%) adverse reactions leading to dose interruption included electrocardiogram QT prolonged (14%) and differentiation syndrome (11%). Two (7%) patients required a dose reduction due to electrocardiogram QT prolonged. One patient each required permanent discontinuation due to diarrhea and PRES.
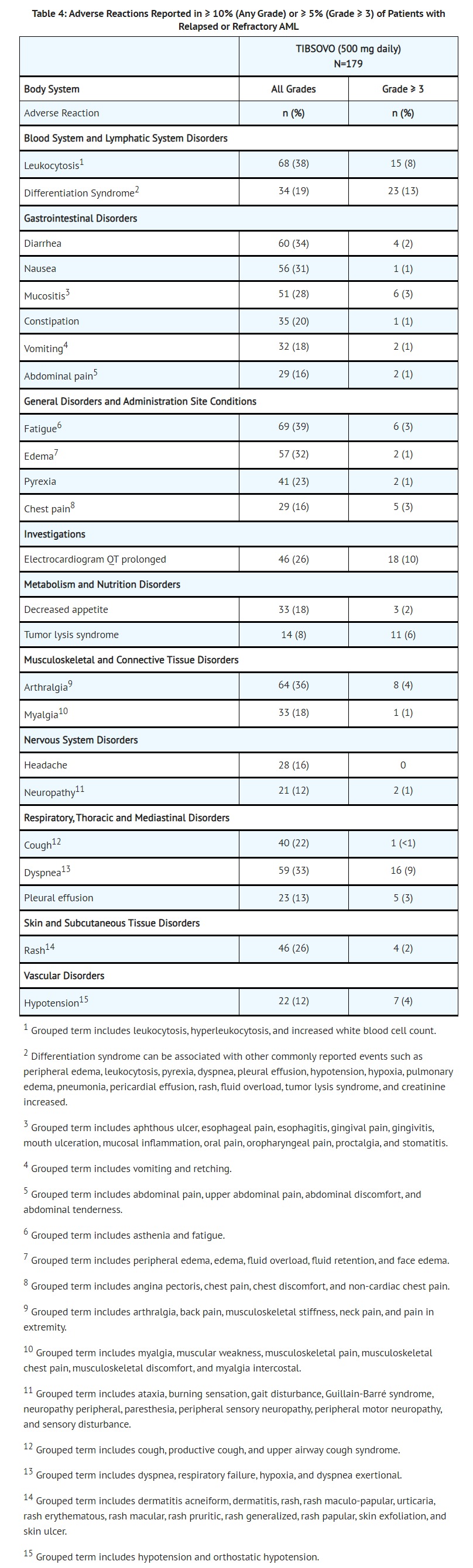
- Relapsed or Refractory AML
- Serious adverse reactions (≥ 5%) were differentiation syndrome (10%), leukocytosis (10%), and electrocardiogram QT prolonged (7%). There was one case of progressive multifocal leukoencephalopathy (PML).
- The most common adverse reactions leading to dose interruption were electrocardiogram QT prolonged (7%), differentiation syndrome (3%), leukocytosis (3%) and dyspnea (3%). Five out of 179 patients (3%) required a dose reduction due to an adverse reaction. Adverse reactions leading to a dose reduction included electrocardiogram QT prolonged (1%), diarrhea (1%), nausea (1%), decreased hemoglobin (1%), and increased transaminases (1%). Adverse reactions leading to permanent discontinuation included Guillain-Barré syndrome (1%), rash (1%), stomatitis (1%), and creatinine increased (1%).
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- A concentration-dependent QTc interval prolongation of approximately 17.2 msec (90% CI: 14.7, 19.7) was observed at the steady-state Cmax following a 500 mg daily dose based on an analysis of 171 patients with advanced hematologic malignances and an IDH1 mutation, including 26 patients with newly diagnosed AML and 136 patients with relapsed or refractory AML, who received TIBSOVO 500 mg daily [see Warnings and Precautions (5.1)]. Co-administration with moderate or strong CYP3A inhibitors is expected to further increase QTc interval prolongation from baseline.
- PATIENT COUNSELING INFORMATION
- QTc Interval Prolongation
- Inform patients of symptoms that may be indicative of significant QTc interval prolongation including dizziness, lightheadedness, and fainting. Advise patients to report these symptoms and the use of all medications to their healthcare provider [see Warnings and Precautions (5.2)].
- MEDICATION GUIDE
- TIBSOVO may cause serious side effects, including:
- Changes in the electrical activity of your heart called QTc prolongation. QTc prolongation can cause irregular heartbeats that can be life-threatening. Your healthcare provider will check the electrical activity of your heart with a test called an electrocardiogram (ECG) during treatment with TIBSOVO. Tell your healthcare provider right away if you feel dizzy, lightheaded, or faint.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
13
24079
Other ADRs
1985
38379602
Odds Ratio = 10.439
Drug Property Information
ATC Code(s):
- L01XX62 - ivosidenib
- L01XX - Other antineoplastic agents
- L01X - OTHER ANTINEOPLASTIC AGENTS
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:IVOSIDENIB
Active Ingredient UNII:Q2PCN8MAM6
Drugbank ID:DB14568
PubChem Compound:N/ADIR Classification
CTD ID:C000627630
CAS Number:1448347-49-6
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 

SMILE Code:
[H][C@@](N(C(=O)[C@]1([H])CCC(=O)N1C1=NC=CC(=C1)C#N)C1=CC(F)=CN=C1)(C(=O)NC1CC(F)(F)C1)C1=C(Cl)C=CC=C1
[H][C@@](N(C(=O)[C@]1([H])CCC(=O)N1C1=NC=CC(=C1)C#N)C1=CC(F)=CN=C1)(C(=O)NC1CC(F)(F)C1)C1=C(Cl)C=CC=C1
Reference
1: Population pharmacokinetic and exposure-response analyses of ivosidenib in patients with IDH1-mutant advanced hematologic malignancies.
[Jiang Xuemin,Wada Russ,Poland Bill,Kleijn Huub Jan,Fan Bin,Liu Guowen,Liu Hua,Kapsalis Stephanie,Yang Hua,Le Kha]Clin Transl Sci,2021 Jan 25. PMID: 33493392
2: Mutant Isocitrate Dehydrogenase 1 Inhibitor Ivosidenib in Combination With Azacitidine for Newly Diagnosed Acute Myeloid Leukemia.
[DiNardo Courtney D,Stein Anthony S,Stein Eytan M,Fathi Amir T,Frankfurt Olga,Schuh Andre C,Döhner Hartmut,Martinelli Giovanni,Patel Prapti A,Raffoux Emmanuel,Tan Peter,Zeidan Amer M,de Botton Stéphane,Kantarjian Hagop M,Stone Richard M,Frattini Mark G,Lersch Frederik,Gong Jing,Gianolio Diego A,Zhang Vickie,Franovic Aleksandra,Fan Bin,Goldwasser Meredith,Daigle Scott,Choe Sung,Wu Bin,Winkler Thomas,Vyas Paresh]J Clin Oncol,2021 Jan 1;39(1):57-65. PMID: 33119479
3: Drug-drug interactions of newly approved small molecule inhibitors for acute myeloid leukemia.
[Megías-Vericat Juan Eduardo,Solana-Altabella Antonio,Ballesta-López Octavio,Martínez-Cuadrón David,Montesinos Pau]Ann Hematol,2020 Sep;99(9):1989-2007. PMID: 32683457
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.