Search for drugs:
Typing the drug name to query
EVEROLIMUS
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- In a randomized, placebo-controlled, cross-over study, 59 healthy subjects were administered a single oral dose of Everolimus (20 mg and 50 mg) and placebo. Everolimus at single doses up to 50 mg did not prolong the QT/QTc interval.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
18
24074
Other ADRs
113324
38268263
Odds Ratio = 0.253
Drug Property Information
ATC Code(s):
- L04AA18 - everolimus
- L04AA1 -
- L04AA - Selective immunosuppressants
- L04A - IMMUNOSUPPRESSANTS
- L04 - IMMUNOSUPPRESSANTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
- L01EG02 - everolimus
- L01EG -
- L01E -
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:EVEROLIMUS
Active Ingredient UNII:9HW64Q8G6G
Drugbank ID:DB01590
PubChem Compound:6442177
CTD ID: D000068338
PharmGKB:PA164746311
CAS Number:159351-69-6
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 1.5 mg/day L04AA18
Chemical Structure: 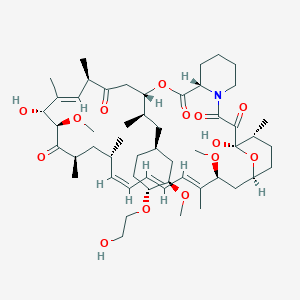

SMILE Code:
[H][C@@]1(C[C@@H](C)[C@]2([H])CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@]3([H])CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@@]3([H])C(=O)O2)OC)CC[C@@H](OCCO)[C@@H](C1)OC
[H][C@@]1(C[C@@H](C)[C@]2([H])CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@]3([H])CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@@]3([H])C(=O)O2)OC)CC[C@@H](OCCO)[C@@H](C1)OC
Reference
1: Phase I/II trial of exemestane, ribociclib, and everolimus in women with HR+/HER2- advanced breast cancer after progression on CDK4/6 inhibitors (TRINITI-1).
[Bardia Aditya,Hurvitz Sara A,DeMichele Angela,Clark Amy S,Zelnak Amelia,Yardley Denise,Karuturi Meghan Sri,Sanft Tara,Blau Sibel,Hart Lowell,Ma Cynthia X,Rugo Hope S,Purkayastha Das,Moulder-Thompson Stacy]Clin Cancer Res,2021 Mar 15;clincanres.2114.2020. PMID: 33722897
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.