Search for drugs:
Typing the drug name to query
EMPAGLIFLOZIN
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- In a randomized, placebo-controlled, active-comparator, crossover study, 30 healthy subjects were administered a single oral dose of JARDIANCE 25 mg, JARDIANCE 200 mg (8 times the maximum dose), moxifloxacin, and placebo. No increase in QTc was observed with either 25 mg or 200 mg empagliflozin.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
1
24091
Other ADRs
32736
38348851
Odds Ratio = 0.049
Drug Property Information
ATC Code(s):
- A10BK03 - empagliflozin
- A10BK -
- A10B - "BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS"
- A10 - DRUGS USED IN DIABETES
- A - ALIMENTARY TRACT AND METABOLISM
- A10BD19 - empagliflozin
- A10BD - Combinations of oral blood glucose lowering drugs
- A10B - "BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS"
- A10 - DRUGS USED IN DIABETES
- A - ALIMENTARY TRACT AND METABOLISM
- A10BD20 - empagliflozin
- A10BD - Combinations of oral blood glucose lowering drugs
- A10B - "BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS"
- A10 - DRUGS USED IN DIABETES
- A - ALIMENTARY TRACT AND METABOLISM
Active Ingredient:empagliflozin
Active Ingredient UNII:HDC1R2M35U
Drugbank ID:DB09038
PubChem Compound:11949646
CTD ID:C570240
PharmGKB:PA166163327
CAS Number:864070-44-0
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 17.5 mg/day A10BK03
Chemical Structure: 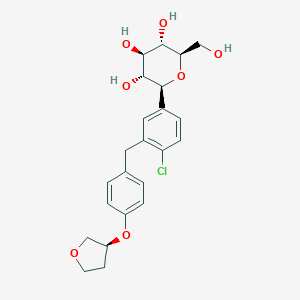

SMILE Code:
OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)C1=CC=C(Cl)C(CC2=CC=C(O[C@H]3CCOC3)C=C2)=C1
OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)C1=CC=C(Cl)C(CC2=CC=C(O[C@H]3CCOC3)C=C2)=C1
Reference
1: Empagliflozin significantly attenuates sotalol-induced QTc prolongation in rats.
[Özgür Barış Veysel,Dinçsoy Berk,Gedikli Esra,Erdemb Ayşen]Kardiol Pol,2021 Jan 25;79(1):53-57. PMID: 33146500
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.