Search for drugs:
Typing the drug name to query
FLUCONAZOLE
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- PRECAUTIONS
- General
- Some azoles, including fluconazole, have been associated with prolongation of the QT interval on the electrocardiogram. During post-marketing surveillance, there have been rare cases of QT prolongation and torsade de pointes in patients taking fluconazole. Most of these reports involved seriously ill patients with multiple confounding risk factors, such as structural heart disease, electrolyte abnormalities and concomitant medications that may have been contributory.
- Fluconazole should be administered with caution to patients with these potentially proarrhythmic conditions.
- Concomitant use of fluconazole and erythromycin has the potential to increase the risk of cardiotoxicity (prolonged QT interval, torsade de pointes) and consequently sudden heart death. This combination should be avoided.
- Fluconazole should be administered with caution to patients with renal dysfunction.
- Fluconazole is a potent CYP2C9 inhibitor and a moderate CYP3A4 inhibitor. Fluconazole treated patients who are concomitantly treated with drugs with a narrow therapeutic window metabolized through CYP2C9 and CYP3A4 should be monitored.
- [Drug Interactions]
- Terfenadine: Because of the occurrence of serious cardiac dysrhythmias secondary to prolongation of the QTc interval in patients receiving azole antifungals in conjunction with terfenadine, interaction studies have been performed. One study at a 200 mg daily dose of fluconazole failed to demonstrate a prolongation in QTc interval. Another study at a 400 mg and 800 mg daily dose of fluconazole demonstrated that fluconazole taken in doses of 400 mg per day or greater significantly increases plasma levels of terfenadine when taken concomitantly. The combined use of fluconazole at doses of 400 mg or greater with terfenadine is contraindicated. (See CONTRAINDICATIONS and CLINICAL PHARMACOLOGY: DRUG INTERACTION STUDIES.) The coadministration of fluconazole at doses lower than 400 mg/day with terfenadine should be carefully monitored.
- Cisapride: There have been reports of cardiac events, including torsade de pointes, in patients to whom fluconazole and cisapride were coadministered. A controlled study found that concomitant fluconazole 200 mg once daily and cisapride 20 mg four times a day yielded a significant increase in cisapride plasma levels and prolongation of QTc interval. The combined use of fluconazole with cisapride is contraindicated. (See CONTRAINDICATIONS and CLINICAL PHARMACOLOGY: DRUG INTERACTION STUDIES.)
- Astemizole: Concomitant administration of fluconazole with astemizole may decrease the clearance of astemizole. Resulting increased plasma concentrations of astemizole can lead to QT prolongation and rare occurrences of torsade de pointes. Coadministration of fluconazole and astemizole is contraindicated.
- Pimozide: Although not studied in vitro or in vivo, concomitant administration of fluconazole with pimozide may result in inhibition of pimozide metabolism. Increased pimozide plasma concentrations can lead to QT prolongation and rare occurrences of torsade de pointes. Coadministration of fluconazole and pimozide is contraindicated.
- Quinidine: Although not studied in vitro or in vivo, concomitant administration of fluconazole with quinidine may result in inhibition of quinidine metabolism. Use of quinidine has been associated with QT prolongation and rare occurrences of torsades de pointes. Coadministration of fluconazole and quinidine is contraindicated. (See CONTRAINDICATIONS. )
- CONTRAINDICATIONS
- Fluconazole is contraindicated in patients who have shown hypersensitivity to fluconazole or to any of its excipients. There is no information regarding cross-hypersensitivity between fluconazole and other azole antifungal agents. Caution should be used in prescribing fluconazole to patients with hypersensitivity to other azoles. Coadministration of terfenadine is contraindicated in patients receiving fluconazole at multiple doses of 400 mg or higher based upon results of a multiple dose interaction study. Coadministration of other drugs known to prolong the QT interval and which are metabolized via the enzyme CYP3A4 such as cisapride, astemizole, erythromycin, pimozide, and quinidine are contraindicated in patients receiving fluconazole. (See CLINICALPHARMACOLOGY: DRUG INTERACTION STUDIESand PRECAUTIONS.)
- ADVERSE REACTIONS
- Post-Marketing Experience
- Cardiovascular: QT prolongation, torsade de pointes. (See PRECAUTIONS.)
- CLINICAL PHARMACOLOGY
- Drug Interaction Studies
- Terfenadine: Six healthy volunteers received terfenadine 60 mg BID for 15 days. Fluconazole 200 mg was administered daily from days 9 through 15. Fluconazole did not affect terfenadine plasma concentrations. Terfenadine acid metabolite AUC increased 36% ± 36% (range: 7 to 102%) from day 8 to day 15 with the concomitant administration of fluconazole. There was no change in cardiac repolarization as measured by Holter QTc intervals. Another study at a 400 mg and 800 mg daily dose of fluconazole demonstrated that fluconazole taken in doses of 400 mg per day or greater significantly increases plasma levels of terfenadine when taken concomitantly. (See CONTRAINDICATIONS and PRECAUTIONS. )
- Quinidine: Although not studied in vitro or in vivo, concomitant administration of fluconazole with quinidine may result in inhibition of quinidine metabolism. Use of quinidine has been associated with QT prolongation and rare occurrences of torsades de pointes. Coadministration of fluconazole and quinidine is contraindicated. (See CONTRAINDICATIONSand PRECAUTIONS.)
- Cisapride: A placebo-controlled, randomized, multiple-dose study examined the potential interaction of fluconazole with cisapride. Two groups of 10 normal subjects were administered fluconazole 200 mg daily or placebo. Cisapride 20 mg four times daily was started after 7 days of fluconazole or placebo dosing. Following a single dose of fluconazole, there was a 101% increase in the cisapride AUC and a 91% increase in the cisapride C max. Following multiple doses of fluconazole, there was a 192% increase in the cisapride AUC and a 154% increase in the cisapride C max. Fluconazole significantly increased the QTc interval in subjects receiving cisapride 20 mg four times daily for 5 days. (See CONTRAINDICATIONSand PRECAUTIONS.)
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
175
23917
Other ADRs
23820
38357767
Odds Ratio = 11.783
Drug Property Information
ATC Code(s):
- J02AC01 - fluconazole
- J02AC - Triazole derivatives
- J02A - ANTIMYCOTICS FOR SYSTEMIC USE
- J02 - ANTIMYCOTICS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:FLUCONAZOLE
Active Ingredient UNII:8VZV102JFY
Drugbank ID:DB00196
PubChem Compound:3365
CTD ID:D015725
PharmGKB:PA449653
CAS Number:86386-73-4
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 200.0 mg/day J02AC01
Chemical Structure: 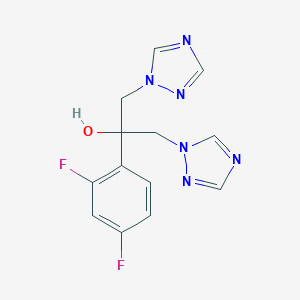

SMILE Code:
OC(CN1C=NC=N1)(CN1C=NC=N1)C1=C(F)C=C(F)C=C1
OC(CN1C=NC=N1)(CN1C=NC=N1)C1=C(F)C=C(F)C=C1
Reference
1: A randomized open label trial of tamoxifen combined with amphotericin B and fluconazole for cryptococcal meningitis.
[Ngan Nguyen Thi Thuy,Mai Nguyen Thi Hoang,Tung Nguyen Le Nhu,Lan Nguyen Phu Huong,Tai Luong Thi Hue,Phu Nguyen Hoan,Chau Nguyen Van Vinh,Binh Tran Quang,Hung Le Quoc,Beardsley Justin,White Nicholas,Lalloo David,Krysan Damian,Hope William,Geskus Ronald,Wolbers Marcel,Nhat Le Thanh Hoang,Thwaites Guy,Kestelyn Evelyne,Day Jeremy]Wellcome Open Res,2019 Jan 22;4:8. PMID: 30801037
2: Concomitant use of levofloxacin and fluconazole leading to possible torsades de pointes.
[Tilton Jessica J,Sadr Rozhan,Groo Vicki L]J Oncol Pharm Pract,2019 Dec;25(8):2004-2006. PMID: 30501378
3: Torsade de pointes and systemic azole antifungal agents: Analysis of global spontaneous safety reports.
[Salem M,Reichlin T,Fasel D,Leuppi-Taegtmeyer A]Glob Cardiol Sci Pract,2017 Jun 30;2017(2):11. PMID: 29644223
4: Isavuconazole shortens the QTc interval.
[Mellinghoff Sibylle C,Bassetti Matteo,Dörfel Daniela,Hagel Stefan,Lehners Nicola,Plis Andrzej,Schalk Enrico,Vena Antonio,Cornely Oliver A]Mycoses,2018 Apr;61(4):256-260. PMID: 29178247
5: QTc prolongation during ciprofloxacin and fluconazole combination therapy: prevalence and associated risk factors.
[Berger Florine A,Monadian Nico,de Groot Natasja M S,Santbergen Bart,van der Sijs Heleen,Becker Matthijs L,Broers Annoek E C,van Gelder Teun,van den Bemt Patricia M L A]Br J Clin Pharmacol,2018 Feb;84(2):369-378. PMID: 29057492
6: Development of a risk score for QTc-prolongation: the RISQ-PATH study.
[Vandael Eline,Vandenberk Bert,Vandenberghe Joris,Spriet Isabel,Willems Rik,Foulon Veerle]Int J Clin Pharm,2017 Apr;39(2):424-432. PMID: 28281228
7: Divergent electrophysiologic profile of fluconazole and voriconazole in an experimental whole-heart model of proarrhythmia.
[Frommeyer Gerrit,Fischer Christina,Lange Philipp S,Leitz Patrick,Fehr Michael,Bogossian Harilaos,Milberg Peter,Eckardt Lars]Eur J Pharmacol,2016 Apr 5;776:185-90. PMID: 26905475
8: [Combination therapy with fluconazole and other QTc-prolonging drugs increase the QTc interval].
[Buch Tina,Andersen Stig Ejdrup]Ugeskr Laeger,2015 Oct 5;177(41):V04150371. PMID: 26471025
9: Extreme doses of intravenous methadone for severe pain in two children with cancer.
[Rasmussen Vinni Faber,Lundberg Vicki,Jespersen Torben Worsøe,Hasle Henrik]Pediatr Blood Cancer,2015 Jun;62(6):1087-90. PMID: 25641929
10: Cutaneous leishmaniasis in Switzerland: first experience with species-specific treatment.
[Mosimann V,Neumayr A,Hatz C,Blum J A]Infection,2013 Dec;41(6):1177-82. PMID: 23835701
11: Effect of combined fluoroquinolone and azole use on QT prolongation in hematology patients.
[Zeuli John D,Wilson John W,Estes Lynn L]Antimicrob Agents Chemother,2013 Mar;57(3):1121-7. PMID: 23229485
12: Torsade de pointes during sevoflurane anesthesia and fluconazole infusion in a patient with long QT syndrome. A case report.
[Tacken M C T,Bracke F A L E,Van Zundert A A J]Acta Anaesthesiol Belg,2011;62(2):105-8. PMID: 21919379
13: Fluconazole inhibits hERG K(+) channel by direct block and disruption of protein trafficking.
[Han Shengna,Zhang Yu,Chen Qiu,Duan Yanyan,Zheng Tenghao,Hu Xiangjie,Zhang Zhao,Zhang Lirong]Eur J Pharmacol,2011 Jan 10;650(1):138-44. PMID: 20951697
14: Long QTc interval and torsade de pointes caused by fluconazole.
[Pham C Phu,de Feiter Peter W,van der Kuy P Hugo M,van Mook Walther Nka]Ann Pharmacother,Jul-Aug 2006;40(7-8):1456-61. PMID: 16849620
15: Two cases of acute promyelocytic leukemia complicated by torsade de pointes during arsenic trioxide therapy.
[Naito Kensuke,Kobayashi Miki,Sahara Naohi,Shigeno Kazuyuki,Nakamura Satoki,Shinjo Kaori,Tobita Tadasu,Takeshita Akihiro,Ohno Ryuzo,Ohnishi Kazunori]Int J Hematol,2006 May;83(4):318-23. PMID: 16757431
16: Torsades de pointes upon fluconazole administration in a patient with acute myeloblastic leukemia.
[Tatetsu Hiro,Asou Norio,Nakamura Miki,Hanaoka Nobuyoshi,Matsuno Fumihiko,Horikawa Kentaro,Mitsuya Hiroaki]Am J Hematol,2006 May;81(5):366-9. PMID: 16628725
17: [Long QT and torsade de pointes in a patient with acquired human immunodeficiency virus infection in multitherapy with drugs affecting cytochrome P450].
[Hrovatin Enzo,Zardo Fabio,Brieda Marco,Dametto Ermanno,Piazza Rita,Antonini-Canterin Francesco,Cassin Matteo,Meneguzzo Nereo,Viel Elda,Lestuzzi Chiara,Di Gennaro Gianpiero,Nicolosi Gian Luigi]Ital Heart J Suppl,2004 Sep;5(9):735-40. PMID: 15568612
18: Fluconazole- and levofloxacin-induced torsades de pointes in an intensive care unit patient.
[Gandhi Pritesh J,Menezes Pearl A,Vu Hien T,Rivera Amy L,Ramaswamy Karthik]Am J Health Syst Pharm,2003 Dec 1;60(23):2479-83. PMID: 14686224
19: Inhibition of cytochrome P450 3A: relevant drug interactions in gastroenterology.
[Sagir A,Schmitt M,Dilger K,Häussinger D]Digestion,2003;68(1):41-8. PMID: 12949438
20: Fluconazole-induced torsade de pointes.
[Tholakanahalli V N,Potti A,Hanley J F,Merliss A D]Ann Pharmacother,2001 Apr;35(4):432-4. PMID: 11302406
21: Drug interactions with cisapride: clinical implications.
[Michalets E L,Williams C R]Clin Pharmacokinet,2000 Jul;39(1):49-75. PMID: 10926350
22: Long QT syndrome and torsade de pointes in a patient receiving fluconazole.
[Wassmann S,Nickenig G,Böhm M]Ann Intern Med,1999 Nov 16;131(10):797. PMID: 10577320
23: Systemic antifungal agents. Drug interactions of clinical significance.
[Albengres E,Le Louët H,Tillement J P]Drug Saf,1998 Feb;18(2):83-97. PMID: 9512916
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.