Search for drugs:
Typing the drug name to query
SUGAMMADEX
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Electrocardiogram QT interval abnormal includes preferred terms electrocardiogram QT interval abnormal and electrocardiogram QT interval prolonged
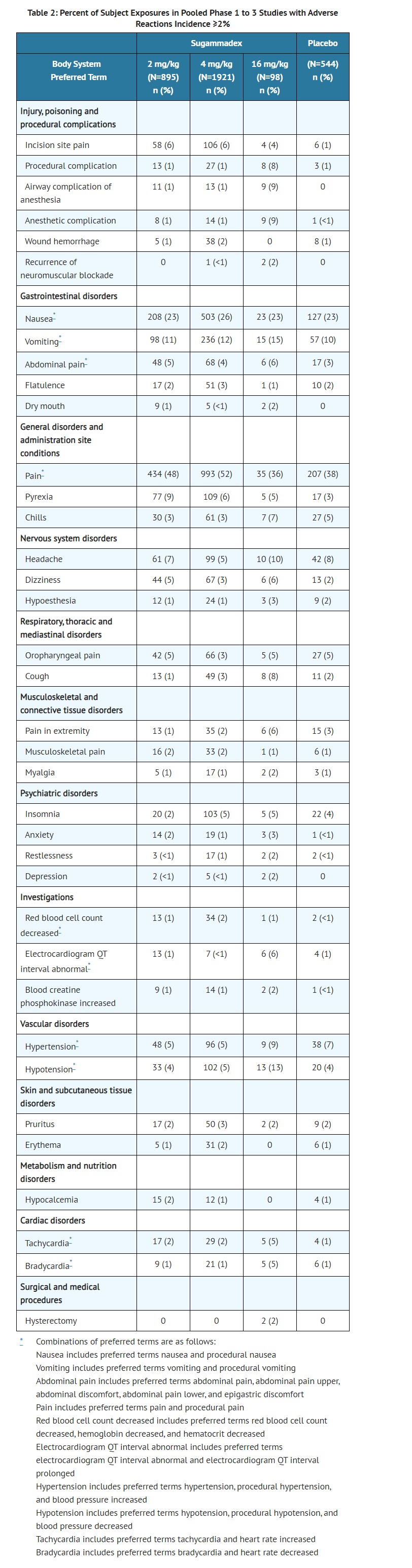
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- At a dose 2 times the maximum recommended dose, sugammadex does not prolong the QTc interval to any clinically relevant extent.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
1
24091
Other ADRs
2341
38379246
Odds Ratio = 0.681
Drug Property Information
ATC Code(s):
- V03AB35 - sugammadex
- V03AB3 -
- V03AB - Antidotes
- V03A - ALL OTHER THERAPEUTIC PRODUCTS
- V03 - ALL OTHER THERAPEUTIC PRODUCTS
- V - VARIOUS
Active Ingredient:SUGAMMADEX SODIUM
Active Ingredient UNII:ERJ6X2MXV7
Drugbank ID:DB06206
PubChem Compound:6918585
CTD ID:D000077122
CAS Number:343306-71-8
Dosage Form(s):injection, solution
Route(s) Of Administrator:intravenous
Daily Dose:
Chemical Structure: 

SMILE Code:
O[C@@H]1[C@@H](O)[C@@H]2O[C@H]3O[C@H](CSCCC(O)=O)[C@@H](O[C@H]4O[C@H](CSCCC(O)=O)[C@@H](O[C@H]5O[C@H](CSCCC(O)=O)[C@@H](O[C@H]6O[C@H](CSCCC(O)=O)[C@@H](O[C@H]7O[C@H](CSCCC(O)=O)[C@@H](O[C@H]8O[C@H](CSCCC(O)=O)[C@@H](O[C@H]9O[C@H](CSCCC(O)=O)[C@@H](O[C@H]1O[C@@H]2CSCCC(O)=O)[C@H](O)[C@H]9O)[C@H](O)[C@H]8O)[C@H](O)[C@H]7O)[C@H](O)[C@H]6O)[C@H](O)[C@H]5O)[C@H](O)[C@H]4O)[C@H](O)[C@H]3O
O[C@@H]1[C@@H](O)[C@@H]2O[C@H]3O[C@H](CSCCC(O)=O)[C@@H](O[C@H]4O[C@H](CSCCC(O)=O)[C@@H](O[C@H]5O[C@H](CSCCC(O)=O)[C@@H](O[C@H]6O[C@H](CSCCC(O)=O)[C@@H](O[C@H]7O[C@H](CSCCC(O)=O)[C@@H](O[C@H]8O[C@H](CSCCC(O)=O)[C@@H](O[C@H]9O[C@H](CSCCC(O)=O)[C@@H](O[C@H]1O[C@@H]2CSCCC(O)=O)[C@H](O)[C@H]9O)[C@H](O)[C@H]8O)[C@H](O)[C@H]7O)[C@H](O)[C@H]6O)[C@H](O)[C@H]5O)[C@H](O)[C@H]4O)[C@H](O)[C@H]3O
Reference
1: Effects of Neostigmine and Sugammadex for Reversal of Neuromuscular Blockade on QT Dispersion Under Propofol Anesthesia: A Randomized Controlled Trial.
[Yamashita Yusuke,Takasusuki Toshifumi,Kimura Yoshiyuki,Komatsuzaki Makoto,Yamaguchi Shigeki]Cardiol Ther,2018 Dec;7(2):163-172. PMID: 30218410
2: Comparison of effects of sugammadex and neostigmine on QTc prolongation in rabbits under general anesthesia.
[Erbaş Mesut,Toman Hüseyin,Şahin Hasan,Kiraz Hasan Ali,Barutcu Ahmet,Simsek Tuncer,Yener Ali Umit,Uzun Metehan,Altinişik Uğur]Acta Cir Bras,2014 Dec;29(12):807-11. PMID: 25517494
3: Impact of anaesthetic drugs and adjuvants on ECG markers of torsadogenicity.
[Staikou C,Stamelos M,Stavroulakis E]Br J Anaesth,2014 Feb;112(2):217-30. PMID: 24305646
4: Sugammadex is not associated with QT/QTc prolongation: methodology aspects of an intravenous moxifloxacin-controlled thorough QT study.
[de Kam Pieter-Jan,van Kuijk Jacqueline,Smeets Jean,Thomsen Torben,Peeters Pierre]Int J Clin Pharmacol Ther,2012 Aug;50(8):595-604. PMID: 22735462
5: Effects of sugammadex doses up to 32 mg/kg alone or in combination with rocuronium or vecuronium on QTc prolongation: a thorough QTc study.
[de Kam Pieter-Jan,van Kuijk Jacqueline,Prohn Marita,Thomsen Torben,Peeters Pierre]Clin Drug Investig,2010;30(9):599-611. PMID: 20568829
6: Reversal of rocuronium-induced neuromuscular blockade with sugammadex in pediatric and adult surgical patients.
[Plaud Benoît,Meretoja Olli,Hofmockel Rainer,Raft Julien,Stoddart Peter A,van Kuijk Jacqueline H M,Hermens Yvonne,Mirakhur Rajinder K]Anesthesiology,2009 Feb;110(2):284-94. PMID: 19194156
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.