Search for drugs:
Typing the drug name to query
CONIVAPTAN HYDROCHLORIDE
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Electrophysiology
- The effect of VAPRISOL 40 mg IV and 80 mg IV on the QT interval was evaluated after the first dose (Day 1) and at the last day during treatment (Day 4) in a randomized, single-blind, parallel group, placebo- and positive-controlled (moxifloxacin 400 mg IV) study in healthy male and female volunteers aged 18 to 45 years. Digital ECGs were obtained at baseline and on Days 1 and 4. Moxifloxacin elicited placebo-corrected changes from baseline in individualized QT correction (QTcI) of +7 to +10 msec on Days 1 and 4, respectively, indicating that the study had assay sensitivity. The placebo-corrected changes from baseline in QTcI in the VAPRISOL 40 mg and 80 mg dose groups on Day 1 were -3.5 msec and -2.9 msec, respectively, and -2.1 msec for both dose groups on Day 4. The results suggest that conivaptan has no clinically significant effect on cardiac repolarization.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
0
38381587
Odds Ratio = N/A
Drug Property Information
ATC Code(s):
- C03XA02 - conivaptan hydrochloride
- C03XA - Vasopressin antagonists
- C03X - OTHER DIURETICS
- C03 - DIURETICS
- C - CARDIOVASCULAR SYSTEM
Active Ingredient:conivaptan hydrochloride
Active Ingredient UNII:75L57R6X36
Drugbank ID:DB00872
PubChem Compound:151171
CTD ID:C106389
PharmGKB:PA164742939
CAS Number:210101-16-9
Dosage Form(s):injection, solution
Route(s) Of Administrator:intravenous
Daily Dose:
Chemical Structure: 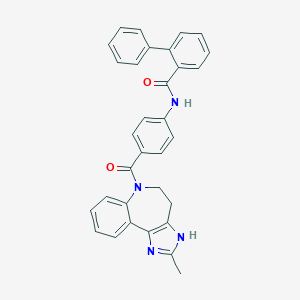

SMILE Code:
CC1=NC2=C(CCN(C(=O)C3=CC=C(NC(=O)C4=CC=CC=C4C4=CC=CC=C4)C=C3)C3=CC=CC=C23)N1
CC1=NC2=C(CCN(C(=O)C3=CC=C(NC(=O)C4=CC=CC=C4C4=CC=CC=C4)C=C3)C3=CC=CC=C23)N1
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.