Search for drugs:
Typing the drug name to query
MILRINONE LACTATE
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Cardiovascular Effects
- Other cardiovascular adverse reactions include hypotension, 2.9% and angina/chest pain, 1.2%.
- In the post-marketing experience, there have been rare cases of “torsades de pointes” reported.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
1
24091
Other ADRs
552
38381035
Odds Ratio = 2.887
Drug Property Information
ATC Code(s):
- C01CE02 - milrinone lactate
- C01CE - Phosphodiesterase inhibitors
- C01C - CARDIAC STIMULANTS EXCL. CARDIAC GLYCOSIDES
- C01 - CARDIAC THERAPY
- C - CARDIOVASCULAR SYSTEM
Active Ingredient:MILRINONE LACTATE
Active Ingredient UNII:9K8XR81MO8
Drugbank ID:DB00235
PubChem Compound:4197
CTD ID:D020105
PharmGKB:PA164749171
CAS Number:78415-72-2
Dosage Form(s):injection, solution
Route(s) Of Administrator:intravenous
Daily Dose:
- 50.0 mg/day C01CE02
Chemical Structure: 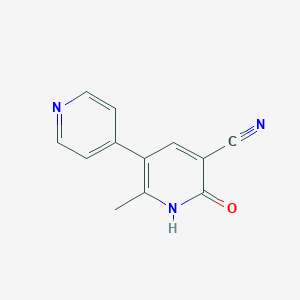

SMILE Code:
CC1=C(C=C(C#N)C(=O)N1)C1=CC=NC=C1
CC1=C(C=C(C#N)C(=O)N1)C1=CC=NC=C1
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.