Search for drugs:
Typing the drug name to query
REMIMAZOLAM BESYLATE
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- In a thorough QT study, 57 healthy volunteers were given an IV push of 10 mg or 20 mg BYFAVO, intravenous midazolam (2.5 mg or 7.5 mg) or placebo, or a single tablet of moxifloxacin 400 mg given orally. The largest mean placebo-adjusted change-from-baseline QTc (upper bound of 2-sided 90% confidence interval) was 6.7 (9.5) ms, 10.7 (13.4) ms, 4.5 (7.3) ms, and 8.1 (10.8) ms, respectively, after treatment with 10 mg or 20 mg BYFAVO, or 2.5 mg or 7.5 mg midazolam.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
0
38381587
Odds Ratio = N/A
Drug Property Information
ATC Code(s):
- N05CD14 - remimazolam besylate
- N05CD - Benzodiazepine derivatives
- N05C - HYPNOTICS AND SEDATIVES
- N05 - PSYCHOLEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:REMIMAZOLAM BESYLATE
Active Ingredient UNII:280XQ6482H
Drugbank ID:DB12404
PubChem Compound:9867812
CTD ID:C522201
CAS Number:308242-62-8
Dosage Form(s):injection, powder, lyophilized, for solution
Route(s) Of Administrator:intravenous
Daily Dose:
Chemical Structure: 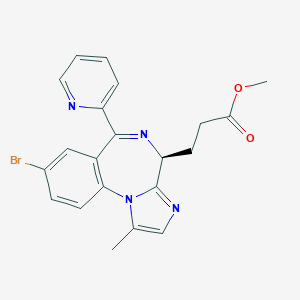

SMILE Code:
COC(=O)CC[C@@H]1N=C(C2=CC=CC=N2)C2=CC(Br)=CC=C2N2C(C)=CN=C12
COC(=O)CC[C@@H]1N=C(C2=CC=CC=N2)C2=CC(Br)=CC=C2N2C(C)=CN=C12
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.