Search for drugs:
Typing the drug name to query
MEXILETINE HYDROCHLORIDE
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- PRECAUTIONS
- Drug Interactions
- Since mexiletine hydrochloride is a substrate for the metabolic pathways involving CYP2D6 and CYP1A2 enzymes, inhibition or induction of either of these enzymes would be expected to alter mexiletine plasma concentrations. In a formal, single-dose interaction study (n = 6 males) the clearance of mexiletine was decreased by 38% following the coadministration of fluvoxamine, an inhibitor of CYP1A2. In another formal study (n = 8 extensive and n = 7 poor metabolizers of CYP2D6), coadministration of propafenone did not alter the kinetics of mexiletine in the poor CYP2D6 metabolizer group. However, the metabolic clearance of mexiletine in the extensive metabolizer phenotype decreased by about 70% making the poor and extensive metabolizer groups indistinguishable. In this crossover steady state study, the pharmacokinetics of propafenone were unaffected in either phenotype by the coadministration of mexiletine. Addition of mexiletine to propafenone did not lead to further electrocardiographic parameters changes of QRS, QTc, RR, and PR intervals than propafenone alone. When concomitant administration of either of these two drugs is initiated with mexiletine, the dose of mexiletine should be slowly titrated to desired effect.
- In a large compassionate use program mexiletine has been used concurrently with commonly employed antianginal, antihypertensive, and anticoagulant drugs without observed interactions. A variety of antiarrhythmics such as quinidine or propranolol were also added, sometimes with improved control of ventricular ectopy. When phenytoin or other hepatic enzyme inducers such as rifampin and phenobarbital have been taken concurrently with mexiletine, lowered mexiletine plasma levels have been reported. Monitoring of mexiletine plasma levels is recommended during such concurrent use to avoid ineffective therapy.
- In a formal study, benzodiazepines were shown not to affect mexiletine plasma concentrations. ECG intervals (PR, QRS, and QT) were not affected by concurrent mexiletine and digoxin, diuretics, or propranolol.
- CLINICAL PHARMACOLOGY
- Electrophysiology in Man
- Mexiletine is a Class 1B antiarrhythmic compound with electrophysiologic properties in man similar to those of lidocaine, but dissimilar from quinidine, procainamide, and disopyramide.
- In patients with normal conduction systems, mexiletine has a minimal effect on cardiac impulse generation and propagation. In clinical trials, no development of second-degree or third-degree AV block was observed. Mexiletine did not prolong ventricular depolarization (QRS duration) or repolarization (QT intervals) as measured by electrocardiography. Theoretically, therefore, mexiletine may be useful in the treatment of ventricular arrhythmias associated with a prolonged QT interval.
- In patients with pre-existing conduction defects, depression of the sinus rate, prolongation of sinus node recovery time, decreased conduction velocity and increased effective refractory period of the intraventricular conduction system have occasionally been observed.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
1150
38380437
Odds Ratio = 0.0
Drug Property Information
ATC Code(s):
- C01BB02 - mexiletine hydrochloride
- C01BB0 -
- C01BB - "Antiarrhythmics, class Ib"
- C01B - "ANTIARRHYTHMICS, CLASS I AND III"
- C01 - CARDIAC THERAPY
- C - CARDIOVASCULAR SYSTEM
Active Ingredient:MEXILETINE HYDROCHLORIDE
Active Ingredient UNII:606D60IS38
Drugbank ID:DB00379
PubChem Compound:4178
CTD ID:D008801
PharmGKB:PA450488
CAS Number:31828-71-4
Dosage Form(s):capsule
Route(s) Of Administrator:oral
Daily Dose:
- 800.0 mg/day C01BB02
Chemical Structure: 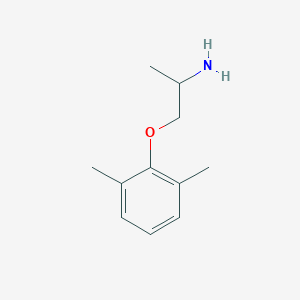

SMILE Code:
CC(N)COC1=C(C)C=CC=C1C
CC(N)COC1=C(C)C=CC=C1C
Reference
1: Long QTc and torsades de pointes in human immunodeficiency virus disease.
[Kocheril A G,Bokhari S A,Batsford W P,Sinusas A J]Pacing Clin Electrophysiol,1997 Nov;20(11):2810-6. PMID: 9392812
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.