Search for drugs:
Typing the drug name to query
VALBENAZINE
DIR Classification
Classification:Moderate-DIQT concern
Severity Score:3.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QT Prolongation
- INGREZZA may prolong the QT interval, although the degree of QT prolongation is not clinically significant at concentrations expected with recommended dosing. In patients taking a strong CYP2D6 or CYP3A4 inhibitor, or who are CYP2D6 poor metabolizers, INGREZZA concentrations may be higher and QT prolongation clinically significant [see Clinical Pharmacology (12.2)]. For patients who are CYP2D6 poor metabolizers or are taking a strong CYP2D6 inhibitor, dose reduction may be necessary. For patients taking a strong CYP3A4 inhibitor, reduce the dose of INGREZZA to 40 mg once daily [see Dosage and Administration (2.3, 2.4)]. INGREZZA should be avoided in patients with congenital long QT syndrome or with arrhythmias associated with a prolonged QT interval. For patients at increased risk of a prolonged QT interval, assess the QT interval before increasing the dosage.
- ADVERSE REACTIONS
- The following adverse reactions are discussed in more detail in other sections of the labeling:
- Hypersensitivity [see Contraindications (4)]
- Somnolence [see Warnings and Precautions (5.1)]
- QT Prolongation [see Warnings and Precautions (5.2)]
- Parkinsonism [see Warnings and Precautions (5.3)]
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- INGREZZA may cause an increase in the corrected QT interval in patients who are CYP2D6 poor metabolizers or who are taking a strong CYP2D6 or CYP3A4 inhibitor. An exposure-response analysis of clinical data from two healthy volunteer studies revealed increased QTc interval with higher plasma concentrations of the active metabolite. Based on this model, patients taking an INGREZZA 80 mg dose with increased exposure to the metabolite (e.g., being a CYP2D6 poor metabolizer) may have a mean QT prolongation of 11.7 msec (14.7 msec upper bound of double-sided 90% CI) as compared to otherwise healthy volunteers given INGREZZA, who had a mean QT prolongation of 6.7 msec (8.4 msec) [see Warnings and Precautions (5.2)].
- PATIENT COUNSELING INFORMATION
- Prolongation of the QT Interval
- Inform patients to consult their physician immediately if they feel faint, lose consciousness, or have heart palpitations [see Warnings and Precautions (5.2)]. Advise patients to inform physicians that they are taking INGREZZA before any new drug is taken.
- PATIENT PACKAGE INSERT
- Before taking INGREZZA, tell your healthcare provider about all of your medical conditions including if you:
- have liver problems
- have heart disease that is not stable, have heart failure or recently had a heart attack
- have an irregular heart rhythm or heartbeat (QT prolongation, heart arrhythmia)
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
27
24065
Other ADRs
24806
38356781
Odds Ratio = 1.735
Drug Property Information
ATC Code(s):
- N07XX13 - valbenazine
- N07XX - Other nervous system drugs
- N07X - OTHER NERVOUS SYSTEM DRUGS
- N07 - OTHER NERVOUS SYSTEM DRUGS
- N - NERVOUS SYSTEM
Active Ingredient:VALBENAZINE
Active Ingredient UNII:54K37P50KH
Drugbank ID:DB11915
PubChem Compound:24795069
CTD ID:C000603978
CAS Number:1025504-45-3
Dosage Form(s):capsule; kit
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 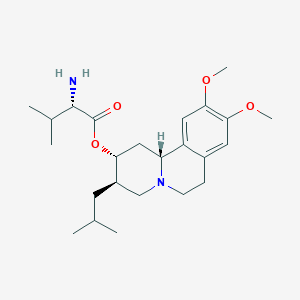

SMILE Code:
COC1=C(OC)C=C2[C@H]3C[C@@H](OC(=O)[C@@H](N)C(C)C)[C@H](CC(C)C)CN3CCC2=C1
COC1=C(OC)C=C2[C@H]3C[C@@H](OC(=O)[C@@H](N)C(C)C)[C@H](CC(C)C)CN3CCC2=C1
Reference
1: Cardiovascular Profile of Valbenazine: Analysis of Pooled Data from Three Randomized, Double-Blind, Placebo-Controlled Trials.
[Thai-Cuarto Dao,O'Brien Christopher F,Jimenez Roland,Liang Grace S,Burke Joshua]Drug Saf,2018 Apr;41(4):429-440. PMID: 29218680
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.