Search for drugs:
Typing the drug name to query
GABAPENTIN ENACARBIL
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- At a dose of 6,000 mg, gabapentin enacarbil does not prolong QTc to a clinically relevant extent.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
75
24017
Other ADRs
176179
38205408
Odds Ratio = 0.678
Drug Property Information
ATC Code(s):
- N03AX12 - gabapentin enacarbil
- N03AX1 -
- N03AX - Other antiepileptics
- N03A - ANTIEPILEPTICS
- N03 - ANTIEPILEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:gabapentin enacarbil
Active Ingredient UNII:75OCL1SPBQ
Drugbank ID:DB08872
PubChem Compound:9883933
CTD ID: C493250
CAS Number:478296-72-9
Dosage Form(s):tablet, extended release
Route(s) Of Administrator:oral
Daily Dose:
- 1800.0 mg/day N03AX12
Chemical Structure: 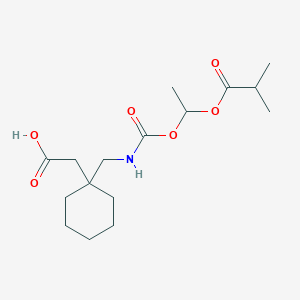

SMILE Code:
CC(C)C(=O)OC(C)OC(=O)NCC1(CC(O)=O)CCCCC1
CC(C)C(=O)OC(C)OC(=O)NCC1(CC(O)=O)CCCCC1
Reference
1: Cardiac repolarization with Gabapentin enacarbil in a randomized, double-blind, placebo- and active-controlled, crossover thorough QT/QTc study in healthy adults.
[Davy Maria,Upward James,Arumugham Thangam,Twomey Colleen,Chen Chao,Stier Brendt]Clin Ther,2013 Dec;35(12):1964-74. PMID: 24290737
2: Evaluation of gabapentin enacarbil on cardiac repolarization: a randomized, double-blind, placebo- and active-controlled, crossover thorough QT/QTc study in healthy adults.
[Chen Dan,Lal Ritu,Zomorodi Katie,Atluri Harisha,Ho Judy,Luo Wendy,Tovera James,Bonzo Daniel,Cundy Kenneth]Clin Ther,2012 Feb;34(2):351-362.e3. PMID: 22325733
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.