Search for drugs:
Typing the drug name to query
HYDROXYZINE HYDROCHLORIDE
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- PRECAUTIONS
- QT Prolongation/Torsade de Pointes (TdP): Cases of QT prolongation and Torsade de Pointes have been reported during post-marketing use of hydroxyzine. The majority of reports occurred in patients with other risk factors for QT prolongation/TdP (pre-existing heart disease, electrolyte imbalances or concomitant arrhythmogenic drug use). Therefore, hydroxyzine should be used with caution in patients with risk factors for QT prolongation, congenital long QT syndrome, a family history of long QT syndrome, other conditions that predispose to QT prolongation and ventricular arrhythmia, as well as recent myocardial infarction, uncompensated heart failure, and bradyarrhythmias.
- Caution is recommended during the concomitant use of drugs known to prolong the QT interval. These include Class 1A (e.g., quinidine, procainamide) or Class III (e.g., amiodarone, sotalol) antiarrhythmics, certain antipsychotics (e.g., ziprasidone, iloperidone, clozapine, quetiapine, chlorpromazine), certain antidepressants (e.g., citalopram, fluoxetine), certain antibiotics (e.g., azithromycin, erythromycin, clarithromycin, gatifloxacin, moxifloxacin); and others (e.g., pentamidine, methadone, ondansetron, droperidol).
- CONTRAINDICATIONS
- Hydroxyzine is contraindicated in patients with a prolonged QT interval.
- OVERDOSAGE
- Hydroxyzine overdose may cause QT prolongation and Torsade de Pointes. ECG monitoring is recommended in cases of hydroxyzine overdose.
- ADVERSE REACTIONS
- Cardiac System
- QT prolongation, Torsade de Pointes.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
102
23990
Other ADRs
9759
38371828
Odds Ratio = 16.718
Drug Property Information
ATC Code(s):
- N05BB01 - hydroxyzine hydrochloride
- N05BB - Diphenylmethane derivatives
- N05B - ANXIOLYTICS
- N05 - PSYCHOLEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:HYDROXYZINE HYDROCHLORIDE
Active Ingredient UNII:76755771U3
Drugbank ID:DB00557
PubChem Compound:3658
CTD ID:D006919
PharmGKB:PA449943
CAS Number:68-88-2
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 75.0 mg/day N05BB01
Chemical Structure: 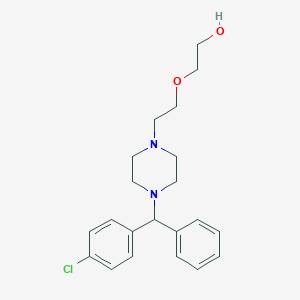

SMILE Code:
OCCOCCN1CCN(CC1)C(C1=CC=CC=C1)C1=CC=C(Cl)C=C1
OCCOCCN1CCN(CC1)C(C1=CC=CC=C1)C1=CC=C(Cl)C=C1
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.