Search for drugs:
Typing the drug name to query
MARAVIROC
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- OVERDOSAGE
- The highest single dose administered in clinical trials was 1,200 mg. The dose-limiting adverse event was postural hypotension, which was observed at 600 mg. While the recommended dose for SELZENTRY in patients receiving a CYP3A inducer without a CYP3A inhibitor is 600 mg twice daily, this dose is appropriate due to enhanced metabolism.
- Prolongation of the QT interval was seen in dogs and monkeys at plasma concentrations 6 and 12 times, respectively, those expected in humans at the intended exposure of 300-mg equivalents twice daily. However, no significant QT prolongation was seen in the trials in treatment-experienced subjects with HIV using the recommended doses of maraviroc, or in a specific pharmacokinetic trial to evaluate the potential of maraviroc to prolong the QT interval [see Clinical Pharmacology (12.2)].
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Effects on Electrocardiogram
- A placebo-controlled, randomized, crossover trial to evaluate the effect on the QT interval of healthy male and female volunteers was conducted with 3 single oral doses of maraviroc and moxifloxacin. The placebo-adjusted mean maximum (upper 1-sided 95% CI) increases in QTc from baseline after 100, 300, and 900 mg of maraviroc were –2 (0), -1 (1), and 1 (3) msec, respectively, and 13 (15) msec for moxifloxacin 400 mg. No subject in any group had an increase in QTc of greater than or equal to 60 msec from baseline. No subject experienced an interval exceeding the potentially clinically relevant threshold of 500 msec.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
2062
38379525
Odds Ratio = 0.0
Drug Property Information
ATC Code(s):
- J05AX09 - maraviroc
- J05AX0 -
- J05AX - Other antivirals
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:MARAVIROC
Active Ingredient UNII:MD6P741W8A
Drugbank ID:DB04835
PubChem Compound:3002977
CTD ID: D000077592
PharmGKB:PA164768835
CAS Number:376348-65-1
Dosage Form(s):solution; tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 600.0 mg/day J05AX09
Chemical Structure: 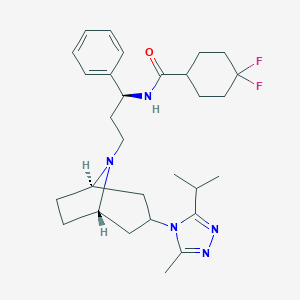

SMILE Code:
CC(C)C1=NN=C(C)N1[C@H]1C[C@@H]2CC[C@H](C1)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)C1=CC=CC=C1
CC(C)C1=NN=C(C)N1[C@H]1C[C@@H]2CC[C@H](C1)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)C1=CC=CC=C1
Reference
1: Maraviroc: new drug. Multiple antiretroviral treatment failure: too soon to reach conclusions.
Prescrire Int,2008 Jun;17(95):98-101. PMID: 18623908
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.