Search for drugs:
Typing the drug name to query
SIPONIMOD
DIR Classification
Classification:Moderate-DIQT concern
Severity Score:3.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- DRUG INTERACTIONS
- MAYZENT has not been studied in patients taking QT prolonging drugs.
- Class Ia (e.g., quinidine, procainamide) and Class III (e.g., amiodarone, sotalol) anti-arrhythmic drugs have been associated with cases of Torsades de Pointes in patients with bradycardia. If treatment with MAYZENT is considered, advice from a cardiologist should be sought.
- Because of the potential additive effects on heart rate, treatment with MAYZENT should generally not be initiated in patients who are concurrently treated with QT prolonging drugs with known arrhythmogenic properties, heart rate lowering calcium channel blockers (e.g., verapamil, diltiazem), or other drugs that may decrease heart rate (e.g., ivabradine, digoxin) [see Warnings and Precautions (5.3) and Drug Interactions (7.3)]. If treatment with MAYZENT is considered, advice from a cardiologist should be sought regarding the switch to non-heart-rate lowering drugs or appropriate monitoring for treatment initiation.
- DOSAGE AND ADMINISTRATION
- First Dose Monitoring in Patients With Certain Preexisting Cardiac Conditions
- If post-dose symptomatic bradycardia, bradyarrhythmia, or conduction related symptoms occur, or if ECG 6 hours postdose shows new onset second-degree or higher AV block or QTc greater than or equal to 500 msec, initiate appropriate management, begin continuous ECG monitoring, and continue monitoring until the symptoms have resolved if no pharmacological treatment is required. If pharmacological treatment is required, continue monitoring overnight and repeat 6-hour monitoring after the second dose.
- Advice from a cardiologist should be sought to determine the most appropriate monitoring strategy (which may include overnight monitoring) during treatment initiation, if treatment with MAYZENT is considered in patients:
- With some preexisting heart and cerebrovascular conditions [see Warnings and Precautions (5.3)]
- With a prolonged QTc interval before dosing or during the 6-hour observation, or at additional risk for QT prolongation, or on concurrent therapy with QT prolonging drugs with a known risk of torsades de pointes [see Warnings and Precautions (5.3) and Drug Interactions (7.2)]
- Receiving concurrent therapy with drugs that slow heart rate or AV conduction [see Drug Interactions (7.2, 7.3)]
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- In a thorough QT study with doses of 2 mg (recommended dose) and 10 mg (five times the recommended dose) siponimod at steady-state, siponimod treatment resulted in a prolongation of QTc , with the maximum mean (upper bound of the two-sided 90% CI) of 7.8 (9.93) ms at 2 mg dose and 7.2 (9.72) ms at 10 mg dose. There was an absence of dose- and exposure-response relationship for QTc effects with the 5-fold dose and exposures achieved by the supratherapeutic dose. No subject had absolute QTcF greater than 480 ms or ΔQTcF greater than 60 ms for siponimod treatment.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
3
24089
Other ADRs
7349
38374238
Odds Ratio = 0.651
Drug Property Information
ATC Code(s):
- L04AA42 - siponimod
- L04AA - Selective immunosuppressants
- L04A - IMMUNOSUPPRESSANTS
- L04 - IMMUNOSUPPRESSANTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:SIPONIMOD
Active Ingredient UNII:RR6P8L282I
Drugbank ID:DB12371
PubChem Compound:44599207
CTD ID:C578989
CAS Number:1230487-00-9
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 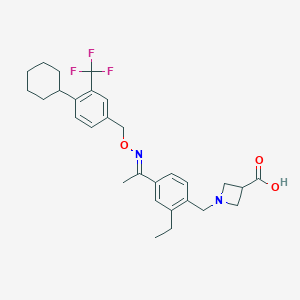

SMILE Code:
CCC1=CC(=CC=C1CN1CC(C1)C(O)=O)C(\C)=N\OCC1=CC=C(C2CCCCC2)C(=C1)C(F)(F)F
CCC1=CC(=CC=C1CN1CC(C1)C(O)=O)C(\C)=N\OCC1=CC=C(C2CCCCC2)C(=C1)C(F)(F)F
Reference
1: Effects of Therapeutic and Supratherapeutic Doses of Siponimod (BAF312) on Cardiac Repolarization in Healthy Subjects.
[Shakeri-Nejad Kasra,Aslanis Vassilios,Veldandi Uday Kiran,Mooney Louise,Pezous Nicole,Brendani Bruno,Juan Axel,Allison Mark,Perry Robert,Legangneux Eric]Clin Ther,2015 Nov 1;37(11):2489-2505.e2. PMID: 26519230
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.