Search for drugs:
Typing the drug name to query
PALONOSETRON HYDROCHLORIDE
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Less common adverse reactions, reported in 1% or less of patients, in Studies 1, 2 and 3 were:
- Cardiovascular: non-sustained tachycardia, bradycardia, hypotension, hypertension, myocardial ischemia, extrasystoles, sinus tachycardia, sinus arrhythmia, supraventricular extrasystoles and QT prolongation.
- [Clinical Trials Experience]
- The most common adverse reactions reported in at least 2% of adults receiving palonosetron 0.075 mg intravenously immediately before induction of anesthesia in 3 randomized placebo-controlled trials [see CLINICAL STUDIES (14.3)] are shown in Table 3. Rates of adverse reactions between palonosetron and placebo groups were similar. Some events are known to be associated with, or may be exacerbated by concomitant perioperative and intraoperative medications administered in this surgical population. A thorough QT/QTc study demonstrated palonosetron does not prolong the QT interval to any clinically relevant extent [see CLINICAL PHARMACOLOGY (12.2)].
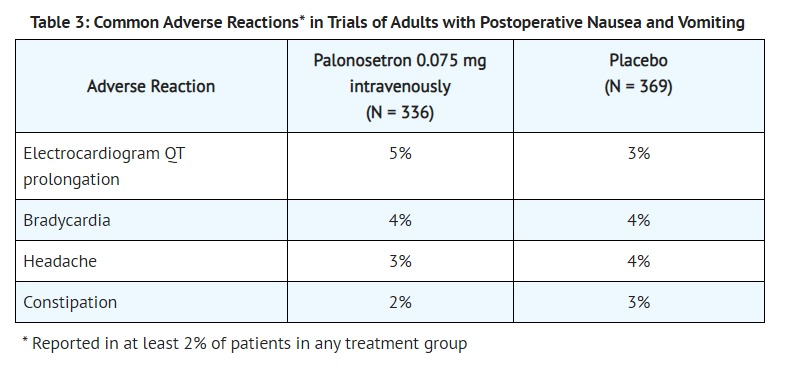
- Less common adverse reactions, reported in 1% of less of patients, in these PONV clinical trials were:
- Cardiovascular: QTc prolongation, sinus bradycardia, tachycardia, blood pressure decreased, hypotension, hypertension, arrhythmia, ventricular extrasystoles, generalized edema, ECG T wave amplitude decreased, platelet count decreased. The frequency of these adverse effects did not appear to be different from placebo.
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- The effect of intravenous palonosetron on blood pressure, heart rate, and ECG parameters including QTc were comparable to intravenous ondansetron and dolasetron in CINV clinical trials. In PONV clinical trials the effect of palonosetron on the QTc interval was no different from placebo. In non-clinical studies palonosetron possesses the ability to block ion channels involved in ventricular de- and re-polarization and to prolong action potential duration.
- At a dose of 9 times the maximum recommended adult dose, palonosetron does not prolong the QT interval to any clinically relevant extent.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
7
24085
Other ADRs
1791
38379796
Odds Ratio = 6.229
Drug Property Information
ATC Code(s):
- A04AA05 - palonosetron hydrochloride
- A04AA - Serotonin (5HT3) antagonists
- A04A - ANTIEMETICS AND ANTINAUSEANTS
- A04 - ANTIEMETICS AND ANTINAUSEANTS
- A - ALIMENTARY TRACT AND METABOLISM
- A04AA55 - palonosetron hydrochloride
- A04AA - Serotonin (5HT3) antagonists
- A04A - ANTIEMETICS AND ANTINAUSEANTS
- A04 - ANTIEMETICS AND ANTINAUSEANTS
- A - ALIMENTARY TRACT AND METABOLISM
Active Ingredient:PALONOSETRON HYDROCHLORIDE
Active Ingredient UNII:23310D4I19
Drugbank ID:DB00377
PubChem Compound:6337614
CTD ID:D000077924
PharmGKB:PA10352
CAS Number:135729-56-5
Dosage Form(s):injection, solution
Route(s) Of Administrator:intravenous
Daily Dose:
- 0.5 mg/day A04AA05
Chemical Structure: 

SMILE Code:
[H][C@]12CCCC3=C1C(=CC=C3)C(=O)N(C2)[C@@H]1CN2CCC1CC2
[H][C@]12CCCC3=C1C(=CC=C3)C(=O)N(C2)[C@@H]1CN2CCC1CC2
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.