Search for drugs:
Typing the drug name to query
ONDANSETRON HYDROCHLORIDE
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QT Prolongation
- Electrocardiogram (ECG) changes including QT interval prolongation have been seen in patients receiving ondansetron. In addition, postmarketing cases of Torsade de Pointes have been reported in patients using ondansetron. Avoid ondansetron in patients with congenital long QT syndrome. ECG monitoring is recommended in patients with electrolyte abnormalities (e.g., hypokalemia or hypomagnesemia), congestive heart failure, bradyarrhythmias, or patients taking other medicinal products that lead to QT prolongation [see CLINICAL PHARMACOLOGY (12.2)] .
- ADVERSE REACTIONS
- Postmarketing Experience
- Cardiovascular
- Arrhythmias (including ventricular and supraventricular tachycardia, premature ventricular contractions, and atrial fibrillation), bradycardia, electrocardiographic alterations (including second-degree heart block, QT/QTc interval prolongation, and ST segment depression), palpitations, and syncope. Rarely and predominantly with intravenous ondansetron, transient ECG changes including QT interval prolongation have been reported.
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- QTc interval prolongation was studied in a double-blind, single-intravenous dose, placebo- and positive- controlled, crossover trial in 58 healthy subjects. The maximum mean (95% upper confidence bound) difference in QTcF from placebo after baseline correction was 19.5 (21.8) milliseconds and 5.6 (7.4) milliseconds after 15- minute intravenous infusions of 32 mg and 8 mg of ondansetron injection, respectively. A significant exposure- response relationship was identified between ondansetron concentration and ΔΔQTcF. Using the established exposure-response relationship, 24 mg infused intravenously over 15 minutes had a mean predicted (95% upper prediction interval) ΔΔQTcF of 14.0 (16.3) milliseconds. In contrast, 16 mg infused intravenously over 15 minutes using the same model had a mean predicted (95% upper prediction interval) ΔΔQTcF of 9.1 (11.2) milliseconds. In this study, the 8 mg dose infused over 15 minutes did not prolong the QT interval to any clinically relevant extent.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
383
23709
Other ADRs
49763
38331824
Odds Ratio = 12.444
Drug Property Information
ATC Code(s):
- A04AA01 - ondansetron hydrochloride
- A04AA - Serotonin (5HT3) antagonists
- A04A - ANTIEMETICS AND ANTINAUSEANTS
- A04 - ANTIEMETICS AND ANTINAUSEANTS
- A - ALIMENTARY TRACT AND METABOLISM
Active Ingredient:ONDANSETRON HYDROCHLORIDE
Active Ingredient UNII:NMH84OZK2B
Drugbank ID:DB00904
PubChem Compound:4595
CTD ID:D017294
PharmGKB:PA450705
CAS Number:99614-02-5
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 16.0 mg/day A04AA01
Chemical Structure: 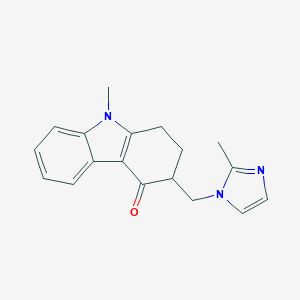

SMILE Code:
CN1C2=C(C3=CC=CC=C13)C(=O)C(CN1C=CN=C1C)CC2
CN1C2=C(C3=CC=CC=C13)C(=O)C(CN1C=CN=C1C)CC2
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.