Search for drugs:
Typing the drug name to query
PRIMAQUINE PHOSPHATE
DIR Classification
Classification:Moderate-DIQT concern
Severity Score:2.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- PRECAUTIONS
- Potential Prolongation of QT Interval
- Due to potential for QT interval prolongation, monitor ECG when using primaquine in patients with cardiac disease, long QT syndrome, a history of ventricular arrhythmias, uncorrected hypokalemia and/or hypomagnesemia, or bradycardia (<50 bpm), and during concomitant administration with QT interval prolonging agents (see PRECAUTIONS, DRUG INTERACTIONS, ADVERSE REACTIONS, and OVERDOSAGE).
- [Drug Interactions]
- Caution is advised if Primaquine is used concomitantly with other drugs that prolong the QT interval (see PRECAUTIONS, ADVERSE REACTIONS, and OVERDOSAGE).
- OVERDOSAGE
- Symptoms of overdosage of primaquine phosphate include abdominal cramps, vomiting, burning epigastric distress, central nervous system and cardiovascular disturbances, including cardiac arrhythmia and QT interval prolongation, cyanosis, methemoglobinemia, moderate leukocytosis or leukopenia, and anemia in G6PD deficient patients. The most striking symptoms are granulocytopenia and acute hemolytic anemia in sensitive persons. Acute hemolysis occurs, but patients recover completely if the dosage is discontinued.
- ADVERSE REACTIONS
- Cardiac: Cardiac Arrhythmia and QT interval prolongation (see PRECAUTIONS, OVERDOSAGE).
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
5
24087
Other ADRs
272
38381315
Odds Ratio = 29.292
Drug Property Information
ATC Code(s):
- P01BA03 - primaquine phosphate
- P01BA - Aminoquinolines
- P01B - ANTIMALARIALS
- P01 - ANTIPROTOZOALS
- P - "ANTIPARASITIC PRODUCTS, INSECTICIDES AND REPELLENTS"
Active Ingredient:PRIMAQUINE PHOSPHATE
Active Ingredient UNII:H0982HF78B
Drugbank ID:DB01087
PubChem Compound:4908
CTD ID:D011319
PharmGKB:PA451103
CAS Number:90-34-6
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 15.0 mg/day P01BA03
Chemical Structure: 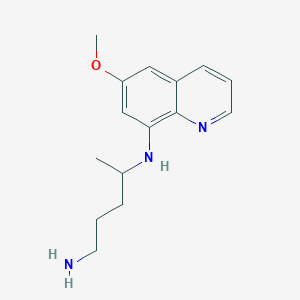

SMILE Code:
COC1=CC(NC(C)CCCN)=C2N=CC=CC2=C1
COC1=CC(NC(C)CCCN)=C2N=CC=CC2=C1
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.