Search for drugs:
Typing the drug name to query
CEFTAROLINE FOSAMIL
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- In a randomized, positive- and placebo-controlled crossover thorough QTc study, 54 healthy subjects were each administered a single dose of Teflaro 1500 mg, placebo, and a positive control by IV infusion over 1 hour. At the 1500 mg dose of Teflaro, no significant effect on QTc interval was detected at peak plasma concentration or at any other time.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
1116
38380471
Odds Ratio = 0.0
Drug Property Information
ATC Code(s):
- J01DI02 - ceftaroline fosamil
- J01DI - Other cephalosporins
- J01D - OTHER BETA-LACTAM ANTIBACTERIALS
- J01 - ANTIBACTERIALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:CEFTAROLINE FOSAMIL
Active Ingredient UNII:7P6FQA5D21
Drugbank ID:DB06590
PubChem Compound:9852981
CTD ID:C515501
CAS Number:229016-73-3
Dosage Form(s):powder, for solution
Route(s) Of Administrator:intravenous
Daily Dose:
- 1200.0 mg/day J01DI02
Chemical Structure: 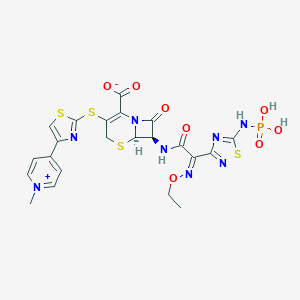

SMILE Code:
[H][C@]12SCC(SC3=NC(=CS3)C3=CC=[N+](C)C=C3)=C(N1C(=O)[C@H]2NC(=O)C(=N/OCC)\C1=NSC(NP(O)(O)=O)=N1)C([O-])=O
[H][C@]12SCC(SC3=NC(=CS3)C3=CC=[N+](C)C=C3)=C(N1C(=O)[C@H]2NC(=O)C(=N/OCC)\C1=NSC(NP(O)(O)=O)=N1)C([O-])=O
Reference
1: Randomized, placebo-controlled study to assess the impact on QT/QTc interval of supratherapeutic doses of ceftazidime-avibactam or ceftaroline fosamil-avibactam.
[Das Shampa,Armstrong Jon,Mathews David,Li Jianguo,Edeki Timi]J Clin Pharmacol,2014 Mar;54(3):331-40. PMID: 24150927
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.