Search for drugs:
Typing the drug name to query
BROMOCRIPTINE MESYLATE
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Clinical Trials Experience
- Syncope
- In clinical trials, syncope was reported in 1.4% of the 2,500 CYCLOSET-treated patients and 0.6% of the 1,454 placebo-treated patients. Among the 3,070 patients studied in the 52-week safety trial, 33 CYCLOSET-treated patients (1.6%) and 7 placebo-treated patients (0.7%) reported syncope. In this trial, electrocardiograms were not available at the time of these events, but an assessment of routine electrocardiograms obtained during the course of the trial did not identify arrhythmias or QTc interval prolongation among the CYCLOSET-treated patients reporting syncope.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
2
24090
Other ADRs
5036
38376551
Odds Ratio = 0.633
Drug Property Information
ATC Code(s):
- G02CB01 - bromocriptine mesylate
- G02CB0 -
- G02CB - Prolactine inhibitors
- G02C - OTHER GYNECOLOGICALS
- G02 - OTHER GYNECOLOGICALS
- G - GENITO URINARY SYSTEM AND SEX HORMONES
- N04BC01 - bromocriptine mesylate
- N04BC - Dopamine agonists
- N04B - DOPAMINERGIC AGENTS
- N04 - ANTI-PARKINSON DRUGS
- N - NERVOUS SYSTEM
Active Ingredient:bromocriptine mesylate
Active Ingredient UNII:FFP983J3OD
Drugbank ID:DB01200
PubChem Compound:31101
CTD ID:D001971
PharmGKB:PA448671
CAS Number:25614-03-3
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 40.0 mg/day N04BC01
- 5.0 mg/day G02CB01
Chemical Structure: 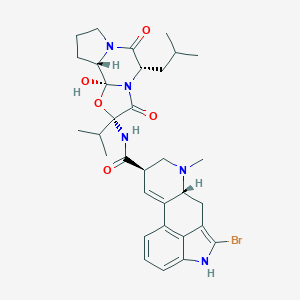

SMILE Code:
[H][C@@]12CCCN1C(=O)[C@H](CC(C)C)N1C(=O)[C@](NC(=O)[C@H]3CN(C)[C@]4([H])CC5=C(Br)NC6=CC=CC(=C56)C4=C3)(O[C@@]21O)C(C)C
[H][C@@]12CCCN1C(=O)[C@H](CC(C)C)N1C(=O)[C@](NC(=O)[C@H]3CN(C)[C@]4([H])CC5=C(Br)NC6=CC=CC(=C56)C4=C3)(O[C@@]21O)C(C)C
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.