Search for drugs:
Typing the drug name to query
BRIVARACETAM
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- At a dose 4 times the maximum recommended dose, BRIVIACT did not prolong the QT interval to a clinically relevant extent.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
0
38381587
Odds Ratio = N/A
Drug Property Information
ATC Code(s):
- N03AX23 - brivaracetam
- N03AX23 -
- N03AX2 -
- N03AX - Other antiepileptics
- N03A - ANTIEPILEPTICS
- N03 - ANTIEPILEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:brivaracetam
Active Ingredient UNII:U863JGG2IA
Drugbank ID:DB05541
PubChem Compound:9837243
CTD ID:C482793
PharmGKB:PA166153491
CAS Number:357336-20-0
Dosage Form(s):injection, suspension; solution; tablet, film coated
Route(s) Of Administrator:intravenous; oral
Daily Dose:
- 100.0 mg/day N03AX23
Chemical Structure: 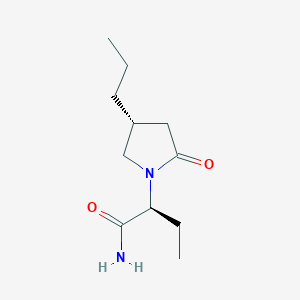

SMILE Code:
CCC[C@H]1CN([C@@H](CC)C(N)=O)C(=O)C1
CCC[C@H]1CN([C@@H](CC)C(N)=O)C(=O)C1
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.