Search for drugs:
Typing the drug name to query
DRONEDARONE
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QT Interval Prolongation
- Dronedarone induces a moderate (average of about 10 ms but much greater effects have been observed) QTc (Bazett) prolongation [see CLINICAL PHARMACOLOGY (12.2), CLINICAL STUDIES (14.1)]. If the QTc Bazett interval is ≥500 ms, discontinue MULTAQ [see CONTRAINDICATIONS (4)].
- [Renal Impairment and Failure]
- Marked increase in serum creatinine, pre-renal azotemia and acute renal failure, often in the setting of heart failure [see WARNINGS AND PRECAUTIONS (5.4)] or hypovolemia, have been reported in patients taking MULTAQ. In most cases, these effects appear to be reversible upon drug discontinuation and with appropriate medical treatment. Monitor renal function periodically.
- Small increases in creatinine levels (about 0.1 mg/dL) following dronedarone treatment initiation have been shown to be a result of inhibition of creatinine's tubular secretion. The elevation has a rapid onset, reaches a plateau after 7 days and is reversible after discontinuation.
- DRUG INTERACTIONS
- Pharmacodynamic Interactions
- Drugs Prolonging the QT Interval (Inducing Torsade de Pointes)
- Coadministration of drugs prolonging the QT interval (such as certain phenothiazines, tricyclic antidepressants, certain macrolide antibiotics, and Class I and III antiarrhythmics) is contraindicated because of the potential risk of torsade de pointes–type ventricular tachycardia [see CONTRAINDICATIONS (4), CLINICAL PHARMACOLOGY (12.3)].
- CONTRAINDICATIONS
- MULTAQ is contraindicated in patients with:
- Concomitant use of strong CYP3A inhibitors, such as ketoconazole, itraconazole, voriconazole, cyclosporine, telithromycin, clarithromycin, nefazodone, and ritonavir [see DRUG INTERACTIONS (7.2)]
- Concomitant use of drugs or herbal products that prolong the QT interval and might increase the risk of torsade de pointes, such as phenothiazine antipsychotics, tricyclic antidepressants, certain oral macrolide antibiotics, and Class I and III antiarrhythmics
- Liver or lung toxicity related to the previous use of amiodarone
- QTc Bazett interval ≥500 ms or PR interval >280 ms
- ADVERSE REACTIONS
- Clinical Trials Experience
- In clinical trials, premature discontinuation because of adverse reactions occurred in 11.8% of the dronedarone-treated patients and in 7.7% of the placebo-treated group. The most common reasons for discontinuation of therapy with MULTAQ were gastrointestinal disorders (3.2% vs 1.8% in the placebo group) and QT prolongation (1.5% vs 0.5% in the placebo group).
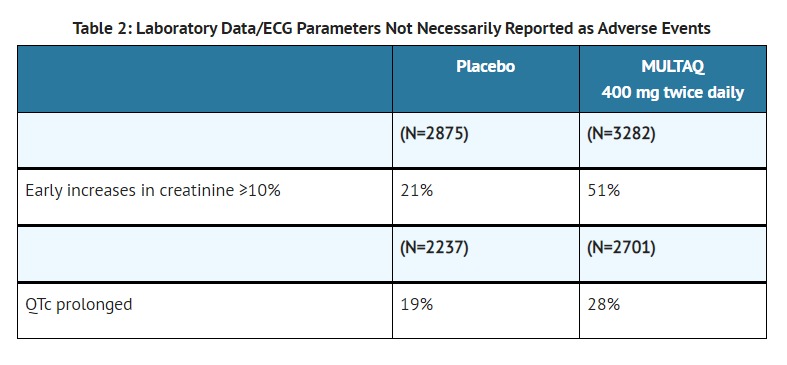
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Electrophysiological Effects
- Dronedarone exhibits properties of all four Vaughn-Williams antiarrhythmic classes, although it is unclear which of these are important in producing dronedarone's clinical effects. The effect of dronedarone on 12-lead ECG parameters (heart rate, PR, and QTc) was investigated in healthy subjects following repeated oral doses up to 1600 mg once daily or 800 mg twice daily for 14 days and 1600 mg twice daily for 10 days. In the dronedarone 400 mg twice-daily group, there was no apparent effect on heart rate; a moderate heart rate lowering effect (about 4 bpm) was noted at 800 mg twice daily. There was a clear dose-dependent effect on PR-interval with an increase of +5 ms at 400 mg twice daily and up to +50 ms at 1600 mg twice daily. There was a moderate dose related effect on the QTc-interval with an increase of +10 ms at 400 mg twice daily and up to +25 ms with 1600 mg twice daily.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
72
24020
Other ADRs
15500
38366087
Odds Ratio = 7.42
Drug Property Information
ATC Code(s):
- C01BD07 - dronedarone
- C01BD - "Antiarrhythmics, class III"
- C01B - "ANTIARRHYTHMICS, CLASS I AND III"
- C01 - CARDIAC THERAPY
- C - CARDIOVASCULAR SYSTEM
Active Ingredient:Dronedarone
Active Ingredient UNII:JQZ1L091Y2
Drugbank ID:DB04855
PubChem Compound:208898
CTD ID: D000077764
PharmGKB:PA153619853
CAS Number:141626-36-0
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 800.0 mg/day C01BD07
Chemical Structure: 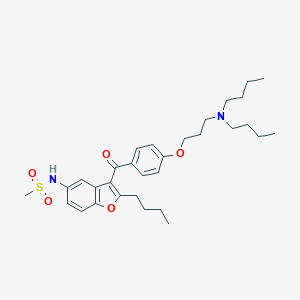

SMILE Code:
CCCCN(CCCC)CCCOC1=CC=C(C=C1)C(=O)C1=C(CCCC)OC2=C1C=C(NS(C)(=O)=O)C=C2
CCCCN(CCCC)CCCOC1=CC=C(C=C1)C(=O)C1=C(CCCC)OC2=C1C=C(NS(C)(=O)=O)C=C2
Reference
1: {'sub': ['Na,L', 'Kr'], '#text': 'In vivo characterization of anti-atrial fibrillatory potential and pharmacological safety profile of I plus I inhibitor ranolazine using the halothane-anesthetized dogs.'}
[Nunoi Yoshio,Kambayashi Ryuichi,Goto Ai,Hagiwara-Nagasawa Mihoko,Chiba Koki,Izumi-Nakaseko Hiroko,Kawai Shinichi,Takei Yoshinori,Matsumoto Akio,Watanabe Yoshinori,Sugiyama Atsushi]Heart Vessels,2021 Mar 24. PMID: 33763729
2: Use of Disproportionality Analysis to Identify Previously Unknown Drug-Associated Causes of Cardiac Arrhythmias Using the Food and Drug Administration Adverse Event Reporting System (FAERS) Database.
[Moreland-Head Lindsay N,Coons James C,Seybert Amy L,Gray Matthew P,Kane-Gill Sandra L]J Cardiovasc Pharmacol Ther,2021 Jan 6;1074248420984082. PMID: 33403858
3: Transtelephonic ECG Monitoring to Guide Outpatient Antiarrhythmic Drug Therapy in Patients With Non-Permanent Atrial Fibrillation: Efficacy and Safety From a Single-Center Experience.
[Klingenheben Thomas,Albakri Aref,M Helms Thomas]J Atr Fibrillation,2019 Apr 30;11(6):2161. PMID: 31384368
4: In vivo Analysis of the Anti-atrial Fibrillatory, Proarrhythmic and Cardiodepressive Profiles of Dronedarone as a Guide for Safety Pharmacological Evaluation of Antiarrhythmic Drugs.
[Motokawa Yoshiyuki,Nakamura Yuji,Hagiwara-Nagasawa Mihoko,Goto Ai,Chiba Koki,Lubna Nur Jaharat,Izumi-Nakaseko Hiroko,Ando Kentaro,Naito Atsuhiko T,Yamazaki Hiroshi,Sugiyama Atsushi]Cardiovasc Toxicol,2018 Jun;18(3):242-251. PMID: 29139031
5: Torsade de pointes tachycardia in a patient on dronedarone therapy.
[Huemer Martin,Sarganas Giselle,Bronder Elisabeth,Klimpel Andreas,Garbe Edeltraut,Haverkamp Wilhelm]Pharmacotherapy,2015 May;35(5):e61-5. PMID: 25823967
6: [Pharmacotherapy of cardiac arrhythmias in women--what do we know, do we have a choice?].
[Klocek Marek,Skrzek Agnieszka,Czarnecka Danuta]Przegl Lek,2014;71(3):155-9. PMID: 25154213
7: Ventricular ectopy and QTc-interval prolongation associated with dronedarone therapy.
[Gonzalez Jaime E,Sauer William H,Krantz Mori J]Pharmacotherapy,2013 Oct;33(10):e179-81. PMID: 23836583
8: Torsade de pointes due to dronedarone: déjà vu?
[Bauman Jerry L]Pharmacotherapy,2012 Aug;32(8):764-6. PMID: 23307524
9: Proarrhythmic potential of dronedarone: emerging evidence from spontaneous adverse event reporting.
[Kao David P,Hiatt William R,Krantz Mori J]Pharmacotherapy,2012 Aug;32(8):767-71. PMID: 22744806
10: Electrophysiologic profile of dronedarone on the ventricular level: beneficial effect on postrepolarization refractoriness in the presence of rapid phase 3 repolarization.
[Milberg Peter,Frommeyer Gerrit,Uphaus Timo,Kaiser Dennis,Osada Nani,Breithardt Günter,Eckardt Lars]J Cardiovasc Pharmacol,2012 Jan;59(1):92-100. PMID: 21964157
11: Targeting the arrhythmogenic substrate in atrial fibrillation: focus on structural remodeling.
[Raschi Emanuel,Boriani Giuseppe,De Ponti Fabrizio]Curr Drug Targets,2011 Feb;12(2):263-86. PMID: 21126225
12: Dronedarone. atrial fibrillation: too many questions about long-term adverse effects.
Prescrire Int,2010 Aug;19(108):149-52. PMID: 20939439
13: [Ionic mechanisms of action of class III antiarrhythmic drugs].
[Kuz'min V S,Rozenshtraukh L V]Kardiologiia,2010;50(7):49-61. PMID: 20659045
14: Worsening heart failure in the setting of dronedarone initiation.
[Coons James C,Plauger Kara M,Seybert Amy L,Sokos George G]Ann Pharmacother,2010 Sep;44(9):1496-500. PMID: 20628043
15: Amiodarone as paradigm for developing new drugs for atrial fibrillation.
[Singh Bramah N]J Cardiovasc Pharmacol,2008 Oct;52(4):300-5. PMID: 18841075
16: Relationship among amiodarone, new class III antiarrhythmics, miscellaneous agents and acquired long QT syndrome.
[Riera Andrés Ricardo Pérez,Uchida Augusto Hiroshi,Ferreira Celso,Ferreira Filho Celso,Schapachnik Edgardo,Dubner Sergio,Zhang Li,Moffa Paulo Jorge]Cardiol J,2008;15(3):209-19. PMID: 18651412
17: A review of the investigational antiarrhythmic agent dronedarone.
[Tafreshi Mohammad J,Rowles Joie]J Cardiovasc Pharmacol Ther,2007 Mar;12(1):15-26. PMID: 17495254
18: A benefit-risk assessment of class III antiarrhythmic agents.
[Elming Hanne,Brendorp Bente,Pehrson Steen,Pedersen Ole Dyg,Køber Lars,Torp-Petersen Christian]Expert Opin Drug Saf,2004 Nov;3(6):559-77. PMID: 15500415
19: IKr channel blockers: novel antiarrhythmic agents.
[Lee K,Park J Y,Ryu P D,Kwon L S,Kim H Y]Curr Med Chem Cardiovasc Hematol Agents,2003 Oct;1(3):203-23. PMID: 15326913
20: Dronedarone for prevention of atrial fibrillation: a dose-ranging study.
[Touboul Paul,Brugada Josep,Capucci Alessandro,Crijns Harry J G M,Edvardsson Nils,Hohnloser Stefan H]Eur Heart J,2003 Aug;24(16):1481-7. PMID: 12919771
21: Chronic amiodarone evokes no torsade de pointes arrhythmias despite QT lengthening in an animal model of acquired long-QT syndrome.
[van Opstal J M,Schoenmakers M,Verduyn S C,de Groot S H,Leunissen J D,van Der Hulst F F,Molenschot M M,Wellens H J,Vos M A]Circulation,2001 Nov 27;104(22):2722-7. PMID: 11723026
22: Evaluation of the acute electrophysiologic effects of intravenous dronedarone, an amiodarone-like agent, with special emphasis on ventricular repolarization and acquired torsade de pointes arrhythmias.
[Verduyn S C,Vos M A,Leunissen H D,van Opstal J M,Wellens H J]J Cardiovasc Pharmacol,1999 Feb;33(2):212-22. PMID: 10028928
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.