Search for drugs:
Typing the drug name to query
TERBUTALINE SULFATE
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS
- Cardiovascular Effects
- Terbutaline sulfate, like all other beta-adrenergic agonists, can produce a clinically significant cardiovascular effect in some patients as measured by pulse rate, blood pressure, and/or symptoms. Although such effects are uncommon after administration of terbutaline sulfate at recommended doses, if they occur, the drug may need to be discontinued. In addition, beta-agonists have been reported to produce electrocardiogram (ECG) changes, such as flattening of the T wave, prolongation of the QTc interval, and ST segment depression. The clinical significance of these findings is unknown. Therefore, terbutaline sulfate, like all sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
740
38380847
Odds Ratio = 0.0
Drug Property Information
ATC Code(s):
- R03AC03 - terbutaline sulfate
- R03AC - Selective beta-2-adrenoreceptor agonists
- R03A - "ADRENERGICS, INHALANTS"
- R03 - DRUGS FOR OBSTRUCTIVE AIRWAY DISEASES
- R - RESPIRATORY SYSTEM
Active Ingredient:TERBUTALINE SULFATE
Active Ingredient UNII:576PU70Y8E
Drugbank ID:DB00871
PubChem Compound:5403
CTD ID:D013726
PharmGKB:PA451616
CAS Number:23031-25-6
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 2.0 mg/day R03AC03
Chemical Structure: 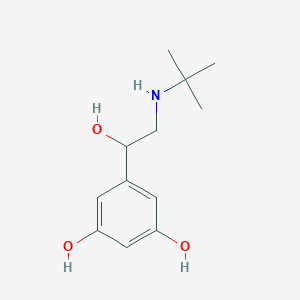

SMILE Code:
CC(C)(C)NCC(O)C1=CC(O)=CC(O)=C1
CC(C)(C)NCC(O)C1=CC(O)=CC(O)=C1
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.