Search for drugs:
Typing the drug name to query
BENDAMUSTINE HYDROCHLORIDE
DIR Classification
Classification:Moderate-DIQT concern
Severity Score:2.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- OVERDOSAGE
- The intravenous LD50 of bendamustine HCl is 240 mg/m2 in the mouse and rat. Toxicities included sedation, tremor, ataxia, convulsions and respiratory distress.
- Across all clinical experience, the reported maximum single dose received was 280 mg/m2. Three of four patients treated at this dose showed ECG changes considered dose-limiting at 7 and 21 days post-dosing. These changes included QT prolongation (one patient), sinus tachycardia (one patient), ST and T wave deviations (two patients) and left anterior fascicular block (one patient). Cardiac enzymes and ejection fractions remained normal in all patients.
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Based on the pharmacokinetics/pharmacodynamics analyses of data from adult NHL patients, nausea increased with increasing bendamustine Cmax.
- Cardiac Electrophysiology
- The effect of bendamustine on the QTc interval was evaluated in 53 patients with indolent NHL and mantle cell lymphoma on Day 1 of Cycle 1 after administration of rituximab at 375 mg/m2 intravenous infusion followed by a 30-minute intravenous infusion of bendamustine at 90 mg/m2/day. No mean changes greater than 20 milliseconds were detected up to one hour post-infusion. The potential for delayed effects on the QT interval after one hour was not evaluated.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
8
24084
Other ADRs
21900
38359687
Odds Ratio = 0.582
Drug Property Information
ATC Code(s):
- L01AA09 - bendamustine hydrochloride
- L01AA - Nitrogen mustard analogues
- L01A - ALKYLATING AGENTS
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:BENDAMUSTINE HYDROCHLORIDE
Active Ingredient UNII:981Y8SX18M
Drugbank ID:DB06769
PubChem Compound:65628
CTD ID:D000069461
CAS Number:16506-27-7
Dosage Form(s):injection, powder, lyophilized, for solution; injection, solution, concentrate
Route(s) Of Administrator:intravenous
Daily Dose:
Chemical Structure: 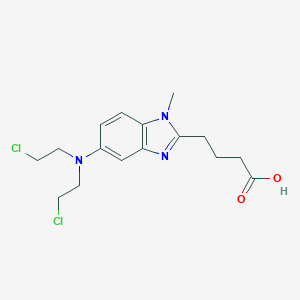

SMILE Code:
CN1C(CCCC(O)=O)=NC2=CC(=CC=C12)N(CCCl)CCCl
CN1C(CCCC(O)=O)=NC2=CC(=CC=C12)N(CCCl)CCCl
Reference
1: Effect of bendamustine in combination with rituximab on QT interval duration in patients with advanced de novo indolent non-Hodgkin or mantle cell lymphoma.
[Burke John M,van der Jagt Richard H C,Flinn Ian W,Craig Michael D,Chen Ling,Morganroth Joel,Munteanu Mihaela C,MacDonald David A]Cancer Chemother Pharmacol,2015 Jul;76(1):211-6. PMID: 26006703
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.