Search for drugs:
Typing the drug name to query
VEMURAFENIB
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QT Prolongation
- Concentration-dependent QT prolongation occurred in an uncontrolled, open-label QT sub-study in previously treated patients with BRAF V600E mutation-positive metastatic melanoma [see CLINICAL PHARMACOLOGY (12.2)]. QT prolongation may lead to an increased risk of ventricular arrhythmias, including Torsade de Pointes.
- Do not start treatment in patients with uncorrectable electrolyte abnormalities, QTc > 500 ms, or long QT syndrome, or in patients who are taking medicinal products known to prolong the QT interval. Prior to and following treatment initiation or after dose modification of ZELBORAF for QTc prolongation, evaluate ECG and electrolytes (including potassium, magnesium, and calcium) after 15 days, monthly during the first 3 months, and then every 3 months thereafter or more often as clinically indicated.
- Withhold ZELBORAF in patients who develop QTc > 500 ms (Grade 3). Upon recovery to QTc ≤ 500 ms (Grade ≤ 2), restart at a reduced dose. Permanently discontinue ZELBORAF treatment if the QTc interval remains > 500 ms and increased > 60 ms from pre-treatment values after controlling cardiac risk factors for QT prolongation (e.g., electrolyte abnormalities, congestive heart failure, and bradyarrhythmias) [see DOSAGE AND ADMINISTRATION (2.3)].
- DOSAGE AND ADMINISTRATION
- Dose Modifications
- Permanently discontinue ZELBORAF for any of the following:
- Grade 4 adverse reaction, first appearance (if clinically appropriate) or second appearance
- QTc prolongation > 500 ms and increased by > 60 ms from pre-treatment values [see WARNINGS AND PRECAUTIONS (5.5)]
- ADVERSE REACTIONS
- Clinical Trials Experience
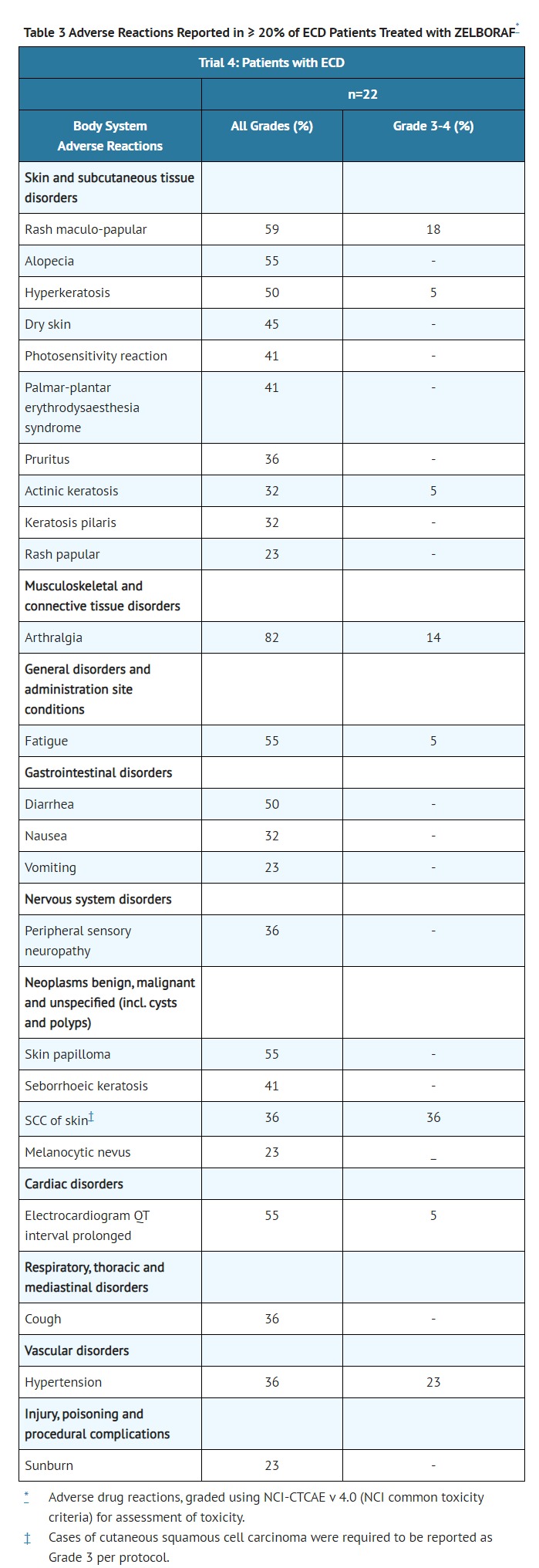
- In Trial 4, the most commonly reported adverse reactions (> 50%) in patients with BRAF V600 mutation- positive ECD treated with ZELBORAF were arthralgia, rash maculo-papular, alopecia, fatigue, electrocardiogram QT interval prolonged, and skin papilloma. The most common (≥ 10%) Grade 3 adverse reactions were squamous cell carcinoma of the skin, hypertension, rash maculo-papular, and arthralgia.
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- In a multi-center, open-label, single-arm study in 132 patients with BRAF V600E mutation-positive metastatic melanoma, patients administered vemurafenib 960 mg orally twice daily did not experience large changes in mean QTc interval (i.e., > 20 ms) from baseline. Vemurafenib is associated with concentration- dependent QTc interval prolongation. The largest mean change from baseline in the first month of treatment occurred at 2 hours post-dose on Day 15—an increase of 12.8 ms (upper boundary of the two-sided 90% confidence interval of 14.9 ms). In the first 6 months of treatment, the largest observed mean change from baseline occurred at a pre-dose time point—an increase of 15.1 ms (upper boundary of the two-sided 90% confidence interval of 17.7 ms).
- [Pharmacokinetics]
- QTc prolongation may occur with increased exposures as vemurafenib is associated with concentration-dependent QTc interval prolongation [see CLINICAL PHARMACOLOGY (12.2)].
- PATIENT COUNSELING INFORMATION
- ZELBORAF can prolong QT interval, which may result in ventricular arrhythmias. Advise patients of the importance of monitoring of their electrolytes and the electrical activity of their heart (via an ECG) during ZELBORAF treatment [see WARNINGS AND PRECAUTIONS (5.5)].
- MEDICATION GUIDE
- Before you take ZELBORAF, tell your healthcare provider about all of your medical conditions, including if you:
- have any heart problems, including a condition called long QT syndromes
- ZELBORAF may cause serious side effects, including:
- Changes in the electrical activity of your heart called QT prolongation. QT prolongation can cause irregular heartbeats that can be life-threatening. Your healthcare provider should do tests before you start taking ZELBORAF and during your treatment with ZELBORAF to check the electrical activity of your heart and your body salts (electrolytes). Tell your healthcare provider right away if you feel faint, lightheaded, dizzy, or feel your heart beating irregularly or fast while taking ZELBORAF. These may be symptoms related to QT prolongation.
- USE IN SPECIFIC POPULATIONS
- Lactation
- There is no information available regarding the presence of vemurafenib in human milk, effects on the breastfed infant, or effects on milk production. Because of the potential for serious adverse reactions in a breastfed infant, including malignancy, severe dermatologic reactions, QT prolongation, hepatotoxicity, photosensitivity, and ophthalmologic toxicity, [see WARNINGS AND PRECAUTIONS (5)], advise women not to breastfeed during treatment with ZELBORAF and for 2 weeks after the final dose.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
85
24007
Other ADRs
26598
38354989
Odds Ratio = 5.106
Drug Property Information
ATC Code(s):
- L01EC01 - vemurafenib
- L01EC -
- L01E -
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:Vemurafenib
Active Ingredient UNII:207SMY3FQT
Drugbank ID:DB08881
PubChem Compound:42611257
CTD ID:D000077484
PharmGKB:PA165946873
CAS Number:918504-65-1
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 

SMILE Code:
CCCS(=O)(=O)NC1=C(F)C(C(=O)C2=CNC3=NC=C(C=C23)C2=CC=C(Cl)C=C2)=C(F)C=C1
CCCS(=O)(=O)NC1=C(F)C(C(=O)C2=CNC3=NC=C(C=C23)C2=CC=C(Cl)C=C2)=C(F)C=C1
Reference
1: Cardiovascular Adverse Events Associated With BRAF and MEK Inhibitors: A Systematic Review and Meta-analysis.
[Mincu Raluca I,Mahabadi Amir A,Michel Lars,Mrotzek Simone M,Schadendorf Dirk,Rassaf Tienush,Totzeck Matthias]JAMA Netw Open,2019 Aug 2;2(8):e198890. PMID: 31397860
2: Tolerability of BRAF/MEK inhibitor combinations: adverse event evaluation and management.
[Heinzerling Lucie,Eigentler Thomas K,Fluck Michael,Hassel Jessica C,Heller-Schenck Daniela,Leipe Jan,Pauschinger Matthias,Vogel Arndt,Zimmer Lisa,Gutzmer Ralf]ESMO Open,2019 May 23;4(3):e000491. PMID: 31231568
3: What links BRAF to the heart function? New insights from the cardiotoxicity of BRAF inhibitors in cancer treatment.
[Bronte Enrico,Bronte Giuseppe,Novo Giuseppina,Bronte Fabrizio,Bavetta Maria Grazia,Lo Re Giuseppe,Brancatelli Giuseppe,Bazan Viviana,Natoli Clara,Novo Salvatore,Russo Antonio]Oncotarget,2015 Nov 3;6(34):35589-601. PMID: 26431495
4: FDA approval summary: vemurafenib for treatment of unresectable or metastatic melanoma with the BRAFV600E mutation.
[Kim Geoffrey,McKee Amy E,Ning Yang-Min,Hazarika Maitreyee,Theoret Marc,Johnson John R,Xu Qiang Casey,Tang Shenghui,Sridhara Rajeshwari,Jiang Xiaoping,He Kun,Roscoe Donna,McGuinn W David,Helms Whitney S,Russell Anne Marie,Miksinski Sarah Pope,Zirkelbach Jeanne Fourie,Earp Justin,Liu Qi,Ibrahim Amna,Justice Robert,Pazdur Richard]Clin Cancer Res,2014 Oct 1;20(19):4994-5000. PMID: 25096067
5: Vemurafenib-induced bilateral facial palsy.
[Shailesh F N U,Singh M,Tiwari U,Hutchins L F]J Postgrad Med,Apr-Jun 2014;60(2):187-8. PMID: 24823520
6: Safety and efficacy of vemurafenib in end stage renal failure.
[Iddawela Mahesh,Crook Sarah,George Leah,Lakkaraju Amit,Nanayakkara Nihal,Hunt Roland,Adam William R]BMC Cancer,2013 Dec 6;13:581. PMID: 24314265
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.