Search for drugs:
Typing the drug name to query
CABOZANTINIB
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- The exposure-response or safety relationship for cabozantinib is unknown.
- Cardiac Electrophysiology
- The effect of cabozantinib on QTc interval was evaluated in a randomized, double-blinded, placebo-controlled trial in patients with medullary thyroid cancer administered a cabozantinib capsule formulation. A mean increase in QTcF of 10 - 15 ms was observed at 4 weeks after initiation. A concentration-QTc relationship could not be definitively established. Changes in cardiac wave form morphology or new rhythms were not observed. No patients in this study had a confirmed QTcF > 500 ms nor did any patients in METEOR, CABOSUN, or CELESTIAL.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
9
24083
Other ADRs
49734
38331853
Odds Ratio = 0.289
Drug Property Information
ATC Code(s):
- L01EX07 - cabozantinib
- L01EX -
- L01E -
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:cabozantinib s-malate
Active Ingredient UNII:DR7ST46X58
Drugbank ID:DB08875
PubChem Compound:25102847
CTD ID: C558660
CAS Number:849217-68-1
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 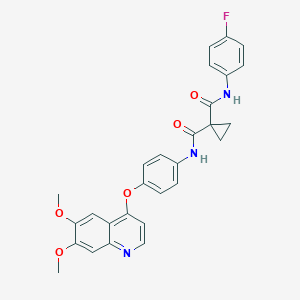

SMILE Code:
COC1=CC2=C(C=C1OC)C(OC1=CC=C(NC(=O)C3(CC3)C(=O)NC3=CC=C(F)C=C3)C=C1)=CC=N2
COC1=CC2=C(C=C1OC)C(OC1=CC=C(NC(=O)C3(CC3)C(=O)NC3=CC=C(F)C=C3)C=C1)=CC=N2
Reference
1: Comparative efficacy and safety of tyrosine kinase inhibitors for thyroid cancer: a systematic review and meta-analysis.
[Oba Takaaki,Chino Tatsunori,Soma Ai,Shimizu Tadafumi,Ono Mayu,Ito Tokiko,Kanai Toshiharu,Maeno Kazuma,Ito Ken-Ichi]Endocr J,2020 Dec 28;67(12):1215-1226. PMID: 32814730
2: Management of treatment-related toxicities in advanced medullary thyroid cancer.
[Brose Marcia S,Bible Keith C,Chow Laura Q M,Gilbert Jill,Grande Carolyn,Worden Francis,Haddad Robert]Cancer Treat Rev,2018 May;66:64-73. PMID: 29704768
3: Update on Cardiovascular Safety of Tyrosine Kinase Inhibitors: With a Special Focus on QT Interval, Left Ventricular Dysfunction and Overall Risk/Benefit.
[Shah Rashmi R,Morganroth Joel]Drug Saf,2015 Aug;38(8):693-710. PMID: 26008987
4: QTc interval prolongation with vascular endothelial growth factor receptor tyrosine kinase inhibitors.
[Ghatalia P,Je Y,Kaymakcalan M D,Sonpavde G,Choueiri T K]Br J Cancer,2015 Jan 20;112(2):296-305. PMID: 25349964
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.