Search for drugs:
Typing the drug name to query
SILODOSIN
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- The effect of silodosin on QT interval was evaluated in a double-blind, randomized, active- (moxifloxacin) and placebo-controlled, parallel-group study in 189 healthy male subjects aged 18 to 45 years. Subjects received either silodosin 8 mg, silodosin 24 mg, or placebo once daily for five days, or a single dose of moxifloxacin 400 mg on Day 5 only. The 24 mg dose of silodosin was selected to achieve blood levels of silodosin that may be seen in a “worst-case” scenario exposure (i.e., in the setting of concomitant renal disease or use of strong CYP3A4 inhibitors) [see Contraindications (4), Warnings and Precautions (5.3) and Clinical Pharmacology (12.3)]. QT interval was measured during a 24-hour period following dosing on Day 5 (at silodosin steady state).
- Silodosin was not associated with an increase in individual corrected (QTcI) QT interval at any time during steady state measurement, while moxifloxacin, the active control, was associated with a maximum 9.59 msec increase in QTcI.
- There has been no signal of Torsade de Pointes in the post-marketing experience with silodosin outside the United States.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
3
24089
Other ADRs
3205
38378382
Odds Ratio = 1.492
Drug Property Information
ATC Code(s):
- G04CA04 - silodosin
- G04CA - Alpha-adrenoreceptor antagonists
- G04C - DRUGS USED IN BENIGN PROSTATIC HYPERTROPHY
- G04 - UROLOGICALS
- G - GENITO URINARY SYSTEM AND SEX HORMONES
Active Ingredient:SILODOSIN
Active Ingredient UNII:CUZ39LUY82
Drugbank ID:DB06207
PubChem Compound:5312125
CTD ID: C095285
PharmGKB:PA165291889
CAS Number:160970-54-7
Dosage Form(s):capsule
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 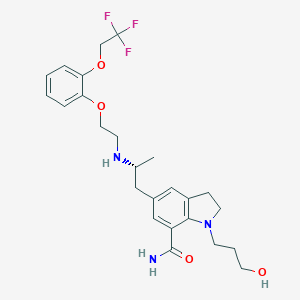

SMILE Code:
C[C@H](CC1=CC2=C(N(CCCO)CC2)C(=C1)C(N)=O)NCCOC1=CC=CC=C1OCC(F)(F)F
C[C@H](CC1=CC2=C(N(CCCO)CC2)C(=C1)C(N)=O)NCCOC1=CC=CC=C1OCC(F)(F)F
Reference
1: A randomized, comparative, open-label study of efficacy and tolerability of alfuzosin, tamsulosin and silodosin in benign prostatic hyperplasia.
[Manjunatha R,Pundarikaksha H P,Madhusudhana H R,Amarkumar J,Hanumantharaju B K]Indian J Pharmacol,Mar-Apr 2016;48(2):134-40. PMID: 27127315
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.