Search for drugs:
Typing the drug name to query
FERUMOXYTOL
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- In a randomized, positive- and placebo-controlled, parallel-group study, healthy subjects received a supratherapeutic regimen of Feraheme (1.02 g given as two 510 mg doses within 24 hours), placebo or a single dose of 400 mg moxifloxacin (positive control). Results demonstrated no effect of Feraheme on QT interval durations. No clinically meaningful effect of Feraheme on heart rate was observed.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
3
24089
Other ADRs
5769
38375818
Odds Ratio = 0.829
Drug Property Information
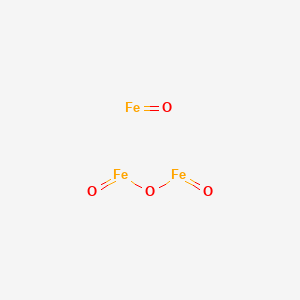
SMILE Code:
[O--].[O--].[O--].[O--].[Fe++].[Fe+3].[Fe+3]
[O--].[O--].[O--].[O--].[Fe++].[Fe+3].[Fe+3]
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.