Search for drugs:
Typing the drug name to query
ITRACONAZOLE
DIR Classification
Classification:Most-DIQT concern
Severity Score:5.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- BOXED WARNING
- Congestive Heart Failure
- TOLSURA can cause or exacerbate congestive heart failure (CHF). When itraconazole was administered intravenously to healthy human volunteers and dogs, negative inotropic effects were seen. If signs or symptoms of congestive heart failure occur or worsen during administration of TOLSURA, reassess the benefit and risk of continuing treatment [see WARNINGS AND PRECAUTIONS (5.1) and ADVERSE REACTIONS (6.1)].
- [Drug Interactions]
- Co-administration of certain drugs that are metabolized by human CYP3A4 enzymes are contraindicated with TOLSURA because plasma concentrations of such drugs are increased, which may also increase or prolong both the pharmacologic effects and/or adverse reactions to these drugs [see CONTRAINDICATIONS (4.1) and DRUG INTERACTIONS (7.1)]
- Co-administration with colchicine, fesoterodine and solifenacin is contraindicated in subjects with varying degrees of renal or hepatic impairment, and
- Co-administration with eliglustat is contraindicated in subjects that are poor or intermediate metabolizers of CYP2D6 and in subjects taking strong or moderate CYP2D6 inhibitors.
- Increased plasma concentrations of some of these drugs caused by co-administration with TOLSURA can lead to QT prolongation and/or ventricular tachyarrhythmias, including occurrences of torsades de pointes, a potentially fatal arrhythmia [see CONTRAINDICATIONS (4.1), WARNINGS AND PRECAUTIONS (5.4) and DRUG INTERACTIONS (7.1)].
- DRUG INTERACTIONS
- Effect of TOLSURA on Other Drugs
- Itraconazole and its major metabolite, hydroxy-itraconazole, are potent CYP3A4 inhibitors. Itraconazole is an inhibitor of the drug transporters P-glycoprotein and breast cancer resistance protein (BCRP). Consequently, itraconazole has the potential to interact with many concomitant drugs resulting in either increased or sometimes decreased concentrations of the concomitant drugs. Increased concentrations may increase the risk of adverse reactions associated with the concomitant drug which can be severe or life-threatening in some cases (e.g., QT prolongation, Torsade de Pointes, respiratory depression, hepatic adverse reactions, hypersensitivity reactions, myelosuppression, hypotension, seizures, angioedema, atrial fibrillation, bradycardia, priapism). Reduced concentrations of concomitant drugs may reduce their efficacy. Table 4 lists examples of drugs that may have their concentrations affected by itraconazole, but is not a comprehensive list. Refer to the approved product labeling to become familiar with the interaction pathways, risk potential, and specific actions to be taken with regards to each concomitant drug prior to initiating therapy with itraconazole.
- CONTRAINDICATIONS
- Drug Interactions
- Increased plasma concentrations of some of these drugs due to co-administration of TOLSURA can lead to QT prolongation and ventricular tachyarrhythmias including occurrences of torsade de pointes, a potentially fatal arrhythmia [see DRUG INTERACTIONS (7.1)].
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
54
24038
Other ADRs
11026
38370561
Odds Ratio = 7.818
Drug Property Information
ATC Code(s):
- J02AC02 - itraconazole
- J02AC - Triazole derivatives
- J02A - ANTIMYCOTICS FOR SYSTEMIC USE
- J02 - ANTIMYCOTICS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:Itraconazole
Active Ingredient UNII:304NUG5GF4
Drugbank ID:DB01167
PubChem Compound:55283
CTD ID:D017964
PharmGKB:PA450132
CAS Number:84625-61-6
Dosage Form(s):capsule, gelatin coated
Route(s) Of Administrator:oral
Daily Dose:
- 200.0 mg/day J02AC02
Chemical Structure: 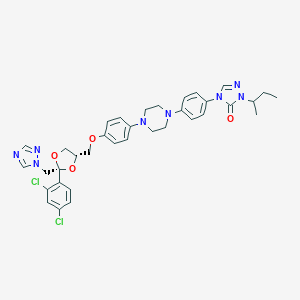

SMILE Code:
CCC(C)N1N=CN(C1=O)C1=CC=C(C=C1)N1CCN(CC1)C1=CC=C(OC[C@H]2CO[C@@](CN3C=NC=N3)(O2)C2=CC=C(Cl)C=C2Cl)C=C1
CCC(C)N1N=CN(C1=O)C1=CC=C(C=C1)N1CCN(CC1)C1=CC=C(OC[C@H]2CO[C@@](CN3C=NC=N3)(O2)C2=CC=C(Cl)C=C2Cl)C=C1
Reference
1: Case Report: QT Prolongation and Abortive Sudden Death Observed in an 85-Year-Old Female Patient With Advanced Lung Cancer Treated With Tyrosine Kinase Inhibitor Osimertinib.
[Kondo Moë,Kisanuki Megumi,Kokawa Yosuke,Gohara Seiichiro,Kawano Osamu,Kagiyama Shuntaro,Maruyama Toru,Odashiro Keita,Maehara Yoshihiko]Front Cardiovasc Med,2021 Mar 19;8:655808. PMID: 33816581
2: PKPD and cardiac single cell modeling of a DDI study with a CYP3A4 substrate and itraconazole to quantify the effects on QT interval duration.
[Jaminion Felix,Bentley Darren,Wang Ken,Wandel Christoph,Derks Michael,Diack Cheikh]J Pharmacokinet Pharmacodyn,2020 Oct;47(5):447-459. PMID: 32572738
3: Treatment of Chronic Pulmonary Aspergillosis: Current Standards and Future Perspectives.
[Alastruey-Izquierdo Ana,Cadranel Jacques,Flick Holger,Godet Cendrine,Hennequin Christophe,Hoenigl Martin,Kosmidis Chris,Lange Christoph,Munteanu Oxana,Page Iain,Salzer Helmut J F,on behalf of CPAnet]Respiration,2018;96(2):159-170. PMID: 29982245
4: Torsade de pointes and systemic azole antifungal agents: Analysis of global spontaneous safety reports.
[Salem M,Reichlin T,Fasel D,Leuppi-Taegtmeyer A]Glob Cardiol Sci Pract,2017 Jun 30;2017(2):11. PMID: 29644223
5: Validation of a population-based method to assess drug-induced alterations in the QT interval: a self-controlled crossover study.
[Iribarren Carlos,Round Alfred D,Peng Jonathan A,Lu Meng,Zaroff Jonathan G,Holve Taylor J,Prasad Amit,Stang Paul]Pharmacoepidemiol Drug Saf,2013 Nov;22(11):1222-32. PMID: 23857878
6: Itraconazole-induced torsade de pointes in a patient receiving methadone substitution therapy.
[NoorZurani Md Haris Robson,Vicknasingam Balasingam,Narayanan Suresh]Drug Alcohol Rev,2009 Nov;28(6):688-90. PMID: 19930027
7: Combined effects of itraconazole and CYP2D6*10 genetic polymorphism on the pharmacokinetics and pharmacodynamics of haloperidol in healthy subjects.
[Park Ji-Young,Shon Ji-Hong,Kim Kyoung-Ah,Jung Hyun-Ju,Shim Joo-Cheol,Yoon Young-Ran,Cha In-June,Shin Jae-Gook]J Clin Psychopharmacol,2006 Apr;26(2):135-42. PMID: 16633141
8: Identification of human p450 isoforms involved in the metabolism of the antiallergic drug, oxatomide, and its inhibitory effect on enzyme activity.
[Goto Akihisa,Adachi Yasuhisa,Inaba Atsuhiro,Nakajima Hiroshi,Kobayashi Hiroyuki,Sakai Kenichi]Biol Pharm Bull,2004 May;27(5):684-90. PMID: 15133245
9: Inhibition of cytochrome P450 3A: relevant drug interactions in gastroenterology.
[Sagir A,Schmitt M,Dilger K,Häussinger D]Digestion,2003;68(1):41-8. PMID: 12949438
10: Effect of oxatomide, an antiallergic agent, on QT interval in dogs.
[Iwamoto K,Ikeda J,Nito M,Kosaka N,Ichikawa S,Ohmori K,Sakai K]Arzneimittelforschung,2001;51(12):971-6. PMID: 11799844
11: [Effect of olopatadine hydrochloride, a novel antiallergic agent, on the QT interval in dogs].
[Iwamoto K,Ikeda J,Nito M,Kosaka N,Ichikawa S,Kobayashi H,Ohmori K]Nihon Yakurigaku Zasshi,2001 Jun;117(6):401-9. PMID: 11436518
12: Drug interactions with cisapride: clinical implications.
[Michalets E L,Williams C R]Clin Pharmacokinet,2000 Jul;39(1):49-75. PMID: 10926350
13: Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition.
[Dresser G K,Spence J D,Bailey D G]Clin Pharmacokinet,2000 Jan;38(1):41-57. PMID: 10668858
14: Systemic antifungal agents. Drug interactions of clinical significance.
[Albengres E,Le Louët H,Tillement J P]Drug Saf,1998 Feb;18(2):83-97. PMID: 9512916
15: [A case of pulmonary aspergillosis effectively treated with itraconazole. Possible interaction of the antimycotic agent with hydroquinidine].
[Cruccu V,Pedretti D,Confalonieri F]Clin Ter,1995 May;146(5):383-9. PMID: 7796571
16: Torsades de pointes induced by nonantiarrhythmic drugs.
[Tran H T]Conn Med1994 May;58(5):291-5. PMID: 7915666
17: Itraconazole affects single-dose terfenadine pharmacokinetics and cardiac repolarization pharmacodynamics.
[Honig P K,Wortham D C,Hull R,Zamani K,Smith J E,Cantilena L R]J Clin Pharmacol1993 Dec;33(12):1201-6. PMID: 8126255
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.